Research Article
Volume 4 Issue 1 - 2022
Monoliths in the mR.N.A. Vaccine Purification Process the Silica Resin and other Composite Materials : the Carbon Content
1IMA ACADEMY marijnskaya applied and industrial chemistry branch italy 29121
2Nano Drug Delivery, (a Product Development Firm), United States
3PhD Assistant Professor Faculty of science University of Tripoli Tripoli/Libya
4Professor, Department of Chemistry, Libya Physical Chemistry, Libyan Authority for Scientific Research, Libya
5IMA academy PRESIDENT RU
2Nano Drug Delivery, (a Product Development Firm), United States
3PhD Assistant Professor Faculty of science University of Tripoli Tripoli/Libya
4Professor, Department of Chemistry, Libya Physical Chemistry, Libyan Authority for Scientific Research, Libya
5IMA academy PRESIDENT RU
*Corresponding Author: Mauro Luisetto, IMA ACADEMY marijnskaya applied and industrial chemistry branch italy 29121.
Received: November 05, 2022; Published: November 16, 2022
Abstract
Aim of this work is to verify the role played in chromatographic technique by innovative MONOLITHS In m R.N.A. VACCINE production: material used , technology and products for large scale production. The fact that a great producer use carbon composite in monoliths is of great interest: carbon fibre reinforcement embedded into epoxy thermoset resin. It is also verified the role played by silica products for chromatographic use and its origin ( syntetic or from natural product like Rice). Related the product from Rice it is used as pharmaceutical eccipient.
Also in this cases other great chemicals producer in its website for SILICA GEL for chromatography report a reference related the production of silica form Rice. (not only syntetic so) Of interest the profile of impurity of this product if used in resin colum for purification biopharmaceutical and in particular the carbon content.
Keywords: Biopharmaceuticals; Purification; Chromatography; Resin; Colums; Monoliths; Silica gel Carbon conten; Graphene; Impurity; Toxicological and regulatory aspects
Introduction
In production of biopharmaceuticals the purification process is one of the main phases. In example for the production of the innovative m R.N.A. VACCINE for covid-19 are used an sequence of Chemical- physical process like TFF tangential flow filtration , followed by a chromatografic separation (affinity or ion exhange) and then treated with an Ultrafiltration/diafiltration process.
Between the various methods of separation was used in past membrane, or more recently magnetic beads separation and today used monoliths of composite materials. If TFF filter are of made of plastic materials the chromatographic resin see the use of silica gel or other Polymer.
In research field, diagnostics field and in small laboratory scale are also used graphene carbon coated (resin and magnetic beads). The silica productive process see or a syntetic process or purification – extraction form variuos natual products like RICE HUSK or diatomea shell.
Various literature report production of silica form rice also of high quality and purity and some producer sell this products as pharma eccipients. But this natural product due by its productive proces that imply high temperature treatement show various level of impurity in exmaple related carbon products (99% silica, 1% graphitic)
So two interesting question can be:
- Silica for RICE , due by its lower cost versus syntetic one, are used in resin for large scale biopharmaceutical production?
- If used this carbon particles of the rice silica can be finded in the impurity of the final product afther the purification process?
In order to give responce to this question it is of interest to verify literature available as well as producers website or patents.
From ijzer AC
From https://labchem-wako.fujifilm.com/europe/category/analysis/column_chromatography/silica_gel_packing_agent/index.html
“Silica gel has a strong physical strength and it is the most widely used as column packing agent for analysis field and the purification in chromatographies such as H.P.L.C., medium and low pressure chromatography, and open column chromatography.
“Silica gel has a strong physical strength and it is the most widely used as column packing agent for analysis field and the purification in chromatographies such as H.P.L.C., medium and low pressure chromatography, and open column chromatography.
Chemical Modification
Chemical modification is to bind various functional groups to the silanol groups on the surface of silica gel to change silica gel functions. C18 modified silica gel (ODS) is an octadecyl group-bound silica gel and is suitable for the reverse-phase chromatography RPC. NH2 modified silica gel is an amin opropyl group-bound silica gel and is suitable for normal-phase, hydrophilic interaction, and ion exchange IE chromatography. Unmodified silica gel is mainly used for normal-phase chromatography.
Chemical modification is to bind various functional groups to the silanol groups on the surface of silica gel to change silica gel functions. C18 modified silica gel (ODS) is an octadecyl group-bound silica gel and is suitable for the reverse-phase chromatography RPC. NH2 modified silica gel is an amin opropyl group-bound silica gel and is suitable for normal-phase, hydrophilic interaction, and ion exchange IE chromatography. Unmodified silica gel is mainly used for normal-phase chromatography.
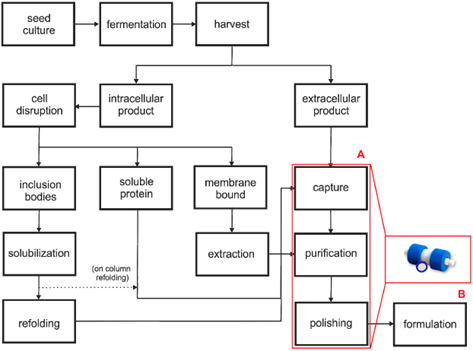
Figure 4: Schematic diagram of a typical bioprocess. (A) different downstream unit operations, (B) convective interaction media (CIM®) monolithic column . From Rajamanickam, V.; Herwig, C.; Spadiut, O. Monoliths in Bioprocess Technology. Chromatography 2015, 2, 195-212. https://doi.org/10.3390/chromatography2020195
Rajamanickam, V.; Herwig, C.; Spadiut, O. Monoliths in Bioprocess Technology. Chromatography 2015, 2, 195-212. https://doi.org/10.3390/chromatography2020195
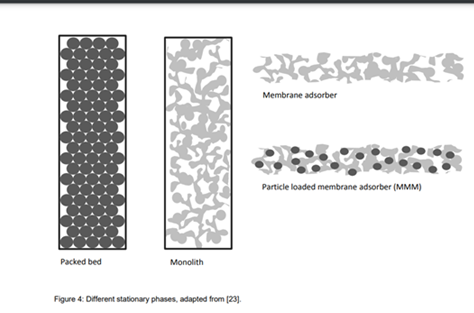
Stationary Phase SP
Monoliths allow a high degree of freedom for the operators, since they can be manufactured from various raw materials (like as polymethacrylate, polyacrylamide, polystyrene, silica, cryogels) with different morphologies and channel diameters. The monolithic media can be classified into 2 categories, namely organic, polymer-based and inorganic, silica-based media. The main advantages of organic, polymer-based media are the pH stability and customizability, but the columns are mechanically not very stable, which shortens the column life-time. Inorganic, silica-based monoliths show an excellent stability and separation efficiency. Manufacturing is sophisticated and also time consuming. Recently in last years, also organic-inorganic hybrid monoliths have been prepared combining the advantages of both.
Monoliths allow a high degree of freedom for the operators, since they can be manufactured from various raw materials (like as polymethacrylate, polyacrylamide, polystyrene, silica, cryogels) with different morphologies and channel diameters. The monolithic media can be classified into 2 categories, namely organic, polymer-based and inorganic, silica-based media. The main advantages of organic, polymer-based media are the pH stability and customizability, but the columns are mechanically not very stable, which shortens the column life-time. Inorganic, silica-based monoliths show an excellent stability and separation efficiency. Manufacturing is sophisticated and also time consuming. Recently in last years, also organic-inorganic hybrid monoliths have been prepared combining the advantages of both.
Commercially Available Monoliths
Monoliths are a great powerful alternative to particle-based resins for the purification of bio molecules, and the first industrial processes employing monoliths have been launched. Several providers offer monoliths of different chemistries, shapes and sizes. Polymethacrylate-based monolithic columns are currently produced/distributed by BIA separations (Slovenia) under the trade name CIM® (convective interaction media), CIMac® (CIM analytical columns) and CIMmultus®. They offer custom-made columns with different column chemistry to suit the needs of the end user. Dionex also produces polymethacrylate monolithic columns under the trade name ProSwift®. Phenomenex (USA) markets silica-based monolithic columns under the trade name Onyx®. The Agilent technologies markets poly-(glycidyl methacrylate-co ethylene dimethacrylate) monoliths, made by BIA separations, under the trade name Bio-monolith®. Merck Millipore (USA) has the trade name Chromolith® for marketing their silica-based monolithic columns. Bio-Rad produces polymer-based monolithic columns under the trade name Uno®.
Monoliths are a great powerful alternative to particle-based resins for the purification of bio molecules, and the first industrial processes employing monoliths have been launched. Several providers offer monoliths of different chemistries, shapes and sizes. Polymethacrylate-based monolithic columns are currently produced/distributed by BIA separations (Slovenia) under the trade name CIM® (convective interaction media), CIMac® (CIM analytical columns) and CIMmultus®. They offer custom-made columns with different column chemistry to suit the needs of the end user. Dionex also produces polymethacrylate monolithic columns under the trade name ProSwift®. Phenomenex (USA) markets silica-based monolithic columns under the trade name Onyx®. The Agilent technologies markets poly-(glycidyl methacrylate-co ethylene dimethacrylate) monoliths, made by BIA separations, under the trade name Bio-monolith®. Merck Millipore (USA) has the trade name Chromolith® for marketing their silica-based monolithic columns. Bio-Rad produces polymer-based monolithic columns under the trade name Uno®.
From ONYX
Especially For The Bio Industry
The Onyx Monolithic C18 in the 150 x 0.1 mm
dimension combines high efficiency, peak capacity, and loadability all in a format for nano-LC
proteomics applications
Carbon Content: 18%
Especially For The Bio Industry
The Onyx Monolithic C18 in the 150 x 0.1 mm
dimension combines high efficiency, peak capacity, and loadability all in a format for nano-LC
proteomics applications
Carbon Content: 18%
From Basics about CIM® technology and key
Applications Aleš Štrancar March, 2011
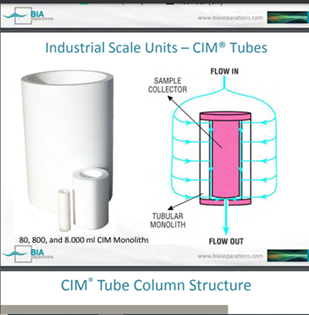
CIMmultus™ from BIA Separations (1 mL – 8 L)
Multiuse Disposable Units - “Plug and Play”
“Carbon fibre reinforcement embedded into epoxy thermoset resin (carbon fibre composite); tough, light material; 5-times lower density than stainless-steel; operate at 20 bar (291 psi).”
Applications Aleš Štrancar March, 2011
CIMmultus™ from BIA Separations (1 mL – 8 L)
Multiuse Disposable Units - “Plug and Play”
“Carbon fibre reinforcement embedded into epoxy thermoset resin (carbon fibre composite); tough, light material; 5-times lower density than stainless-steel; operate at 20 bar (291 psi).”
From Purfication of Nucleic Acid Pete Gagnon
Reverse phase chromatograpy
Reverse phase chromatograpy
From Schmidt, A.; Helgers, H.; Vetter, F.L.; Juckers, A.; Strube, J. Fast and Flexible mRNA Vaccine Manufacturing as a Solution to Pandemic Situations by Adopting Chemical Engineering Good Practice—Continuous Autonomous Operation in Stainless Steel Equipment Concepts. Processes 2021, 9, 1874. https://doi.org/10.3390/pr9111874
“For chromatographic resins, Nuvia aPrime 4a and SiliaSphere C18 were chosen. The resins were chosen based on their separation mechanism, which is hydrophobic anion exchange for aPrime 4a and C18 reversed-phase (RP) for SiliaSphere. Both resins also have large pores of around 1000 Å, allowing pore diffusion to take place. As monoliths, BIA CIMmultusTM PrimaS and Oligo dT18 were modelled”.
Anal Chim Acta. 2009 Oct 12
The use of carrier R.N.A. to enhance D.N.A. extraction from microfluidic-based silica monoliths
Kirsty J Shaw , Lauren Thain, Peter T Docker, C. E Dyer, J. Greenman, G. M Greenway, Stephen J Haswell
“D.N.A. extraction was carried out on silica-based monoliths within a microfluidic device. Solid-phase D.N.A. extraction methodology was applied in which the D.N.A. binds to silica in the presence of a chaotropic salt, such as guanidine hydrochloride, and is eluted in a low ionic strength solution, such as water. The addition of poly-A carrier R.N.A. to the chaotropic salt solution resulted in a marked increase in the effective amount of the D.N.A. that could be recovered (25ng) compared to the absence of R.N.A. (5ng) using the silica-based monolith. These research findings confirm that techniques utilising nucleic acid carrier molecules can enhance D.N.A. extraction methodologies in microfluidic applications”.
The use of carrier R.N.A. to enhance D.N.A. extraction from microfluidic-based silica monoliths
Kirsty J Shaw , Lauren Thain, Peter T Docker, C. E Dyer, J. Greenman, G. M Greenway, Stephen J Haswell
“D.N.A. extraction was carried out on silica-based monoliths within a microfluidic device. Solid-phase D.N.A. extraction methodology was applied in which the D.N.A. binds to silica in the presence of a chaotropic salt, such as guanidine hydrochloride, and is eluted in a low ionic strength solution, such as water. The addition of poly-A carrier R.N.A. to the chaotropic salt solution resulted in a marked increase in the effective amount of the D.N.A. that could be recovered (25ng) compared to the absence of R.N.A. (5ng) using the silica-based monolith. These research findings confirm that techniques utilising nucleic acid carrier molecules can enhance D.N.A. extraction methodologies in microfluidic applications”.
From Journal of Chromatography A Volume 806, Issue 1, 8 May 1998
Preparative purification of supercoiled plasmid D.N.A. using anion-exchange chromatography
Duarte Miguel F Prazeres Thomas Schluep Charles Cooney
https://doi.org/10.1016/S0021-9673(97)01254-5
Preparative purification of supercoiled plasmid D.N.A. using anion-exchange chromatography
Duarte Miguel F Prazeres Thomas Schluep Charles Cooney
https://doi.org/10.1016/S0021-9673(97)01254-5
“Large scale manufacturing of gene vectors such as plasmid D.N.A. is an important issue in gene therapy. Anion-exchange chromatography A.E.C. is fundamental in the downstream processing of plasmids both as a process and analytical technique”
R.N.A. 2013 Oct; 19 Strong anion-exchange fast performance liquid chromatography as a versatile tool for preparation and purification of R.N.A. produced by in vitro transcription Jiri Koubek, K. Feng Lin, Yet R. Chen, R. Ping Cheng, J. Jen Tse Huang “here we demonstrate the use of strong anion-exchange AE fast performance liquid chromatography (FPLC) as a simple, fast, and robust method for R.N.A. production by the in vitro transcription IVT. With this technique, we have purified different transcription templates from unreacted reagents in large quantities. The same buffer system could be used to readily remove nuclease contamination from the over-expressed pyrophosphatase, the important reagent for in vitro transcription.
The method can be used to monitor in vitro transcription reactions to enable facile optimization of reaction conditions, and we have compared the separation performance between strong and weak anion-exchange FPLC for various transcribed R.N.A.s, including the Diels-Alder ribozyme, the hammerhead ribozyme tR.N.A., and 4.5S R.N.A.. The functionality of the purified tR.N.A.(Cys) has been confirmed by the aminoacylation assay. Only the purification by strong anion-exchange F.P.L.C has led to the enrichment of the functional tR.N.A. from run-off transcripts as revealed by both enzymatic and electrophoretic analysis.
Google patent WO2014144767A1
WIPO (PCT)
International application published with inter. search report Ion exchange purification of mR.N.A. “The current landscape for the preparative chromatographic R.N.A. purification uses reversed phase H.P.L.C., but this technique presents many issues with process scale up and ion exchange for preparative purification has only been used for short R.N.A.s. The invention provides preparative purification of R.N.A. ( mR.N.A.) using ion (anion) exchange chromatography AEC that allows for separation of longer R.N.A.s up to 10,000 nucleotides in length via a scalable method. This method avoids problems with current techniques by using low pressure chromatography that is agreeable with existing equipment in cG.M.P commercial facilities, that uses aqueous-bases solutions as the mobile phase (rather than the flammable organic solvents), that uses sorbents displaying binding capacities of greater than 10 mg R.N.A./mL resin (using larger pore sorbents, >500 Angstroms, that display greater mR.N.A. binding capacities), and that yields desired R.N.A. salt forms for downstream DS formulation with no additional manipulation necessary (unlike the ion pair reverse phase techniques). The ion exchange IE sorbent can comprise a positively-charged functional group linked to solid phase media. The ion exchange sorbent used can have a binding capacity of greater than 10 mg R.N.A. transcript/mL sorbent, or greater than 12, 15, 20, 25, 30, 35, 40, 45, 50, 55, 60, 100 mg R.N.A./mL sorbent or higher, or any value or fractional value in between, or any range including or within these numbers.
WIPO (PCT)
International application published with inter. search report Ion exchange purification of mR.N.A. “The current landscape for the preparative chromatographic R.N.A. purification uses reversed phase H.P.L.C., but this technique presents many issues with process scale up and ion exchange for preparative purification has only been used for short R.N.A.s. The invention provides preparative purification of R.N.A. ( mR.N.A.) using ion (anion) exchange chromatography AEC that allows for separation of longer R.N.A.s up to 10,000 nucleotides in length via a scalable method. This method avoids problems with current techniques by using low pressure chromatography that is agreeable with existing equipment in cG.M.P commercial facilities, that uses aqueous-bases solutions as the mobile phase (rather than the flammable organic solvents), that uses sorbents displaying binding capacities of greater than 10 mg R.N.A./mL resin (using larger pore sorbents, >500 Angstroms, that display greater mR.N.A. binding capacities), and that yields desired R.N.A. salt forms for downstream DS formulation with no additional manipulation necessary (unlike the ion pair reverse phase techniques). The ion exchange IE sorbent can comprise a positively-charged functional group linked to solid phase media. The ion exchange sorbent used can have a binding capacity of greater than 10 mg R.N.A. transcript/mL sorbent, or greater than 12, 15, 20, 25, 30, 35, 40, 45, 50, 55, 60, 100 mg R.N.A./mL sorbent or higher, or any value or fractional value in between, or any range including or within these numbers.
The sorbent can also have smaller binding capacities of 1 to 10 mg R.N.A. transcript/mL sorbent. In some embodiments the ion exchange sorbent is a porous media. A variety of different particle and pore sizes can be used. Particle sizes can include the standard sizes used in chromatography methods, including sizes in the range of less than 1 μιη or 1 to 500 μιη ( 5, 10, 20, 50, 75, 100, 150. μιη), or any number or fractional number in between, or any range including or within these numbers. Larger or smaller sizes can also be used. Particles can include the small silica beads or other kinds of particles. Pore sizes can include sizes that are greater than 500 Angstroms, or greater than or equal to 600, 700, 800, 900, 1000, 2000, 4000, or 8000 Angstroms, or any number or fractional number in between, or any range including or within these numbers. Smaller pore sizes can also be used, like as 1 to 500 Angstroms.
The ion exchange IE sorbent can be poly styrene divinylbenzene, polymethacrylate, crosslinked agarose, silica, or allyl dextran with N-N-bis acrylamide, among a variety of other sorbents. The ion exchange sorbent can also be a non-porous media, such as a monolithic column. In some embodiments, membrane-based ion exchangers are used, such as MILLIPORE CHROMASORB™ or SARTORIUS SARTOBIND®. In some embodiments, a mixed mode or combination of ion exchangers can be used. As explained above, the ion exchange sorbent or resin can be strong or weak. The term weak resins includes resins that have a low affinity for polypeptides and an high affinity for polynucleotides, R.N.A. transcripts. The term weak resins also includes resins that have low affinity for polypeptides and a low affinity for polynucleotides, R.N.A. transcripts.
Google pantent US20190203199A1
United States
Polynucleotide purification with monolith columns “Described herein are methods of purifying polynucleotides, imR.N.A. and oligonucleotides, probes, primers and siR.N.A., using monolithic columns with immobilized ligands coupled to the monolithic column MC. Also described are the monolithic columns for purifying polynucleotides from a sample; and methods of preparing such columns. The monolithic matrix may be derived from a variety of materials, such as but not limited to, polymethacrylate, polyacrylamide, polystyrene, silica , cryogels.”
United States
Polynucleotide purification with monolith columns “Described herein are methods of purifying polynucleotides, imR.N.A. and oligonucleotides, probes, primers and siR.N.A., using monolithic columns with immobilized ligands coupled to the monolithic column MC. Also described are the monolithic columns for purifying polynucleotides from a sample; and methods of preparing such columns. The monolithic matrix may be derived from a variety of materials, such as but not limited to, polymethacrylate, polyacrylamide, polystyrene, silica , cryogels.”
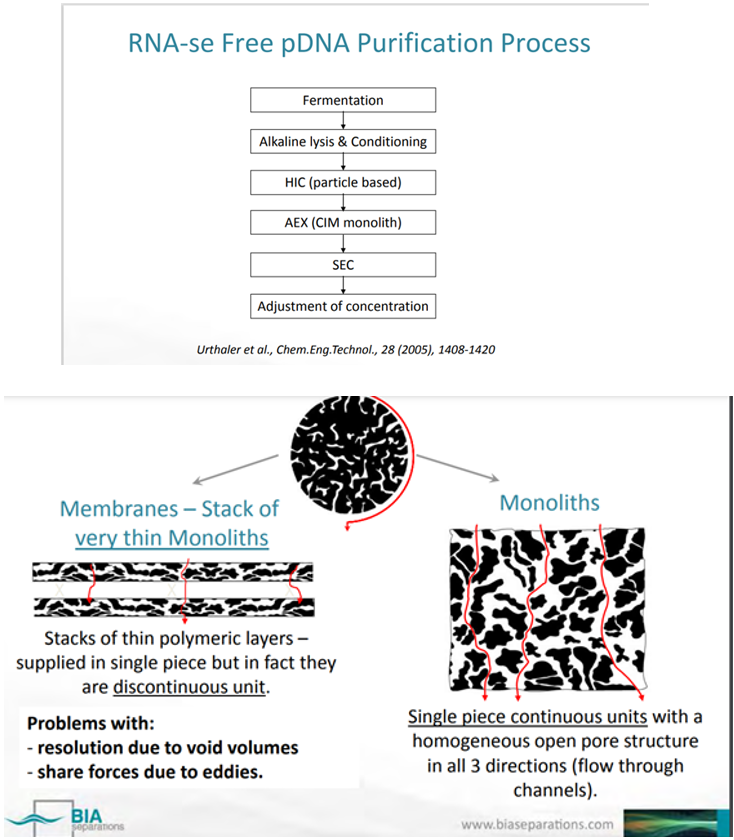
Monoliths for purification of a D.N.A. vaccine against Influenza Diana Stefanía Faria Bicho 2016 “Monoliths are considered the 4th generation of chromatographic stationary phases. They are also called continuous beds, consisting in a single piece of highly porous organic or inorganic solid material into a column in form of disk, rods or tubes. The structure of the monoliths avoids generation of shear forces, thereby contributing to high functional recoveries, even for labile biomolecules such as live virus vaccines, D.N.A. plasmids, and large proteins . This structural feature eliminates some problems related to the scale-up and scale-down variations of conventional matrices, the packing quality and the need to repack a column due to the inadvertent introduction of air bubbles. Monoliths also differ from conventional supports with respect to their specific hydrodynamic properties,”
https://www.sartorius.com/download/1102292/vivaflow-graphene-application-note-en-l-sartorius-pdf-data.pdf
An Improved Method to Wash Graphene Prior to Use as a Drug Delivery Vehicle Abdelnour H. Alhourani, J.Cashman, Klaus Schöne “Due to its ultra-high surface area and ability to be functionalized, graphene is suitable for use in many biomedical applications, including gene and drug delivery.”
An Improved Method to Wash Graphene Prior to Use as a Drug Delivery Vehicle Abdelnour H. Alhourani, J.Cashman, Klaus Schöne “Due to its ultra-high surface area and ability to be functionalized, graphene is suitable for use in many biomedical applications, including gene and drug delivery.”
Material and Methods
Related the topics of this work various relevant literature, patents and other reference from producers are reported and analyzed. All literature comes from scientific biomedical database. After this review part an Experimental project hipotesys is submitted to the researcher in order to produce a global conclusion.
FROM literature:
From Cleaning Chromatography Resin Residues from Surfaces
April 1, 2019
Dijana Hadziselimovic, E. Rivera
From Cleaning Chromatography Resin Residues from Surfaces
April 1, 2019
Dijana Hadziselimovic, E. Rivera
BioPharm International, BioPharm Int.-04-01-2019 “Liquid chromatography LC is used for separating materials in biopharmaceutical production, primarily for purifying proteins by separating product and impurities. The stationary phase SP in liquid chromatography uses fine, solid beads referred to as resins that are packed and held in a column by meshes. These particles can be physically or chemically modified to provide specificity to grab or repel molecules within mixtures. The chemical compatibility allows resins to be stored in caustic solutions, which can be beneficial due to their antimicrobial properties. Lastly, carbon content is variable (but mostly negligible) from resin to resin compared to proteins.”
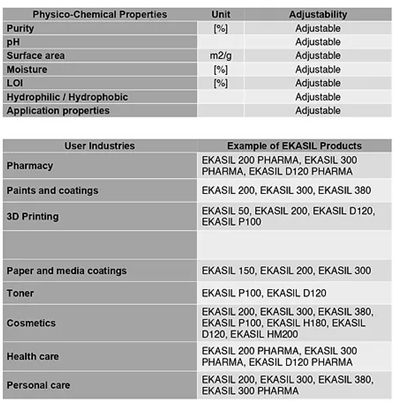
EKASIL Rice Husk Silica
(1) Basic Specifications (2) Advanced Specifications
EKASIL advanced specifications are silica products from rice husk that are developed from the basic specifications based on customers’ requirements of physico-chemical properties. The specifications are various and applicable to a wide range of user industries.
(1) Basic Specifications (2) Advanced Specifications
EKASIL advanced specifications are silica products from rice husk that are developed from the basic specifications based on customers’ requirements of physico-chemical properties. The specifications are various and applicable to a wide range of user industries.
EKASIL 200 PHARMA
Download Physico-Chemical Data
EKASIL Rice Husk Silica
EKASIL 200 PHARMA is high purity silicon dioxide from rice husk for pharmaceutical uses
High purity amorphous silica for pharmaceutical uses in all types of dosage forms
Download Physico-Chemical Data
EKASIL Rice Husk Silica
EKASIL 200 PHARMA is high purity silicon dioxide from rice husk for pharmaceutical uses
High purity amorphous silica for pharmaceutical uses in all types of dosage forms
Free flow and anti-caking agent to improve powder properties Improving tablets properties like as hardness and friability Used as viscosity increasing agent to thicken and thixotropic liquids
Used as anti-setting, thickening and anti-sagging agent High purity, low humidity
Used as anti-setting, thickening and anti-sagging agent High purity, low humidity
No influence of taste Not altering natural colour of powder formulations SK S. Hossain, Lakshya Mathur & P.K. Roy (2018) Rice husk/rice husk ash as an alternative source of silica in ceramics: A review, Journal of Asian Ceramic Societies, DOI: 10.1080/21870764.2018.1539210
“Chemical method High-purity nano-silica has recently found wide-ranging applications in different fields such as pharmaceuticals, dyes, chromatography, drug delivery systems, electronic components, catalysts and adsorbent materials. As a result, demand for the high purity silica is increasing. Combustion-derived R.H.A (without acid or alkali treatment) contain less than 95 wt.% of SiO2, and the remaining part comprises different alkali oxides and impurities. With appropriate acid or alkali treatment of RH/R.H.A., The SiO2 content can be increased in the system to more than 99 wt.%. Several researchers have adopted different chemical routes to derive the high-purity nano-silica.” (1)
From Molecules Review
Silica-Based Monolithic Columns as a Tool in H.P.L.C.—An Overview of Application in Analysis of Active Compounds in Biological Samples Micha? Staniak et al 9 July 2020 “H.P.L.C is a dynamically developing technique widely used in almost all branches of industry and pharmaceutical, chemical, and agri-food investigations, as well as in lab. practice and scientific research. This technique is based on the separation of target compounds from matrix of samples containing other accompanying constituents; The chromatographic column CC filled with the stationary phase where the separation process takes place is named “the heart of chromatographic system”. Columns with various types of fillings are commercially available; Spherical packed columns are still most commonly used. The historical background of all monolithic columns was brilliantly presented by Svec et al.
Silica-Based Monolithic Columns as a Tool in H.P.L.C.—An Overview of Application in Analysis of Active Compounds in Biological Samples Micha? Staniak et al 9 July 2020 “H.P.L.C is a dynamically developing technique widely used in almost all branches of industry and pharmaceutical, chemical, and agri-food investigations, as well as in lab. practice and scientific research. This technique is based on the separation of target compounds from matrix of samples containing other accompanying constituents; The chromatographic column CC filled with the stationary phase where the separation process takes place is named “the heart of chromatographic system”. Columns with various types of fillings are commercially available; Spherical packed columns are still most commonly used. The historical background of all monolithic columns was brilliantly presented by Svec et al.
Monolithic stationary phases were the subject of the interest for many research groups over the last 30 years. They are often named “monolithic rods” or “silica rods” in the case of silica monolithic columns. Due to the characteristic structure that distinguishes them from traditional spherical fillings and their numerous advantages, including a low susceptibility to clogging and a low flow resistance, they are a very interesting alternative for many scientists. Considering the type of material used for synthesis, monolithic columns can be divided into 2 groups; the first is based on the silica gel and the second is based on polymeric materials. Vyviurska et al. presented an exhaustive comparison of both types of commercially available monoliths.
The major disadvantage of most polymeric monolithic fillings is their inability to separate small molecules; hence, their significance in the analysis of samples with complex matrix such as plant material is low.
They are mostly applied for analysis of compounds with high molecular weight such as proteins or polynucleotides and they have greater importance in electro-chromatographic techniques. Although polymeric monolithic columns are produced by some manufacturers such as BIA Separations (Ljubljana, Slovenia), Bio-Rad Laboratories, or Thermo Scientific (Dionex Corporation) (USA), the majority of reports concern home-made fillings and, in these cases, the reproducibility of results is difficult to obtain because the process of synthesis conducted by different researchers may slightly differ.
Many published studies showed the applicability potential of silica-based monolithic columns in investigations of various samples, including plants, food, dietary supplements, and drugs. So far, numerous review papers described the analytical use of the monolithic columns . Namera et al. showed applications of different types of commercially available silica-based monolithic columns in the analysis of the active compounds in biological materials.
It is worth noting that the Chromolith® Performance RP-18e column (100 × 4.6 mm) was used most commonly. Maruška et al. Researcher presented possible applications of monolithic equipment in phytochemical analysis. Monolithic columns are also widely used in proteomics and metabolomics. Rigobello-Masini et al. presented detailed information on the potential applications of this kind of chromatographic filling in this research area. The aim of our study work is to summarize and update the possible applications of this type of fillings. The review covers papers published after 2006 and focuses on commercially available columns, as, due to the complexity and diversity of the manufacturing process, the batch-to-batch reproducibility of home-made fillings is poor. Currently nowadays , 2 companies produce monolithic columns based on silica for H.P.L.C.—Merck KGaA ( Germany) and Phenomenex (Torrance, CA, USA). Their products are available under trade names Chromolith® and Onyx™, respectively. “(2)
ESTABLISHMENT OF RICE HUSK BY-PRODUCT AS PHARMACEUTICAL EXCIPIENTS
Anil Kumar, D. P. Patel et al Published 2018 “Rice husk RU is a value added material for pharmaceuticals because the rice husk produce significant role as producing cellulose, which used as an excipients in pharmaceuticals. Rice husk has such compatible properties, which can enhance the disintegration process with optimum required at reach to that of standard level of pharmaceutical disintegrating agents. Rice husk extracted celluloses are previously used as disintegrating agents in various pharmaceuticals.” (3)
Silica from Rice as New Drug Delivery Systems March 2017 DOI: 10.5772/66723
Anil Kumar, D. P. Patel et al Published 2018 “Rice husk RU is a value added material for pharmaceuticals because the rice husk produce significant role as producing cellulose, which used as an excipients in pharmaceuticals. Rice husk has such compatible properties, which can enhance the disintegration process with optimum required at reach to that of standard level of pharmaceutical disintegrating agents. Rice husk extracted celluloses are previously used as disintegrating agents in various pharmaceuticals.” (3)
Silica from Rice as New Drug Delivery Systems March 2017 DOI: 10.5772/66723
In book: Rice - Technology and Production Carmen Salazar et al “The pharmaceutical industry PI has seen an increased need of carriers or excipients design that allows the controlled release of a drug in the human body. The main role of an excipient is to carry the drug for its administration under therapeutic index. Between the new generation of excipients, the ordered mesoporous silica (M.S.) presents several advantages, such as excellent biocompatibility, good adsorption capacity, and precise control in the drug delivery. The high cost of synthesis of mesoporous silica restricts its use to industrial applications; A low-cost procedure is necessary for widespread use. Biogenic silica from rice husk (SiO2-rice) could be a new choice as a drug delivery system DDS . This silica is obtained from an acid leaching of rice husk followed by calcinations processes at low temperatures; these conditions produce silica with good adsorption properties, similar to those of M.S. In consequence, the excipient behavior of SiO2-rice was assessed using folic acid as the model drug, displaying an 18.5% of absorption in the SiO2-rice pores, while MS absorbed around 19%. The drug release profiles were similar for both the silicas, suggesting that SiO2-rice could be a low-cost, similar yield excipient for drugs similar to the folic acid.”(4)
J Biomed Biotechnol. 2009 Nov 30
D.N.A., R.N.A., and Protein Extraction: The Past and The Present
Siun Chee Tan,B.Chin Yiap “Silica Matrices —The basis for most of the products related to nucleic acid purification is the unique properties of silica matrices for selective D.N.A. binding. Types of silica materials including glass particles, such as glass powder, silica particles, and glass microfibers prepared by grinding glass fiber filter papers, and including diatomaceous earth. Hydrated silica matrix, which was prepared by refluxing the silicon dioxide in sodium hydroxide or potassium hydroxide at a molar ratio of about 2:1 to 10:1 for at least about 48 hours, had been introduced in D.N.A. purification. D.N.A. binds to the inorganic matrix and is released in heated water.
D.N.A., R.N.A., and Protein Extraction: The Past and The Present
Siun Chee Tan,B.Chin Yiap “Silica Matrices —The basis for most of the products related to nucleic acid purification is the unique properties of silica matrices for selective D.N.A. binding. Types of silica materials including glass particles, such as glass powder, silica particles, and glass microfibers prepared by grinding glass fiber filter papers, and including diatomaceous earth. Hydrated silica matrix, which was prepared by refluxing the silicon dioxide in sodium hydroxide or potassium hydroxide at a molar ratio of about 2:1 to 10:1 for at least about 48 hours, had been introduced in D.N.A. purification. D.N.A. binds to the inorganic matrix and is released in heated water.
The principle of silica matrices purification SMP is based on high affinity of the negatively charged D.N.A. backbone towards the positively charged silica particles . Sodium plays a role as a cation bridge that attracts the negatively charged oxygen in the phosphate backbone of nucleic acid . Sodium cations break the hydrogen bonds between the hydrogen in water and the negatively charged oxygen ions in silica under high salt conditions (pH ≤7). The D.N.A. is tightly bound, and extensive washing removes all contaminations. The purified D.N.A. molecules can be eluted under low ionic strength (pH ≥7) later by using TE buffer or distilled water.
Besides silica matrices, nitrocellulose and polyamide membranes PM like as nylon matrices are also known to bind with nucleic acids, but with less specificity. These materials are often used as solid-phase nucleic acid transfer and hybridization matrices. Polyamide matrices are more durable than nitrocellulose and are known to bind nucleic acids NA irreversibly. Nucleic acids can be immobilized on polyamide matrices in the low ionic strength buffer. (2) Glass Particle powder and beads are useful for nucleic acid purification. D.N.A. isolation from agarose gels involved the use of chaotropic salts to facilitate binding of D.N.A. to common silicate glass, flint glass, and borosilicate glass (glass fiber filter). The adsorption of nucleic acid onto the glass substrate occurs most likely based on the mechanism and principle that similar to adsorption chromatography . Nucleic acid purification NAP can also be done on the silica gel and glass mixture . This invention has discovered that a mixture of silica gel and the glass particles can be used to separate nucleic acid from other substances in the presence of chaotropic salts solution. Magnetic Bead Based Nucleic Acid Purification —Magnetic separation MS is an simple and efficient way which is used in purification of nucleic acid nowadays. Many magnetic carriers are now commercially available. Particles having a magnetic charge may be removed by using a permanent magnet in application of a magnetic field. Often, magnetic carriers with immobilized affinity ligands or prepared from biopolymer showing affinity to the target nucleic acid are used for the isolation process.
Magnetic particles that are produced from different synthetic polymers, biopolymers, porous glass or magnetic particles based on inorganic magnetic materials such as surface-modified iron oxide. Materials with a large surface area are preferred to be used in the binding of nucleic acids NA. Besides silica matrices, nitrocellulose and polyamide membranes like as nylon matrices are also known to bind with nucleic acids, but with less specificity.
Besides silica matrices, nitrocellulose and polyamide membranes such as nylon matrices are also known to bind with nucleic acids, but with less specificity. These materials are often used like as solid-phase nucleic acid transfer and hybridization matrices. Polyamide matrices are more durable than the nitrocellulose and are known to bind nucleic acids irreversibly. Nucleic acids can be immobilized on polyamide matrices in low ionic strength buffer . “5”
Current Tools for Industrial Purification of mR.N.A. An affinity method for R.N.A. purification already exists. Hybridization-affinity chromatography with an oligo dT (poly-thymidine) ligand captures mR.N.A. by its poly-A tail. About 250 mM sodium chloride suppresses electrostatic repulsion between the negatively charged backbone phosphatidic acid residues on the ligand and on R.N.A. That enables them to approach each other closely. R.N.A. is captured by A-T base pairing. Removing the salt reestablishes their mutual charge repulsion, overwhelms A-T hydrogen bonding, and elutes the R.N.A.
Hybridization-affinity with oligo dT can be used for capture, but it bears some limitations. It cannot discriminate ssR.N.A. from dsR.N.A., and it has no ability to fractionate mR.N.A. according to size. Intact product, incomplete transcripts, oligomers, fragments, aggregates; any species with an accessible poly-A tail elutes with all the rest. Oligo dT can be cleaned with 100 mM NaOH, but higher concentrations are not recommended. This is a concern because of the high fouling potential of IVT mixtures.
Figure Rreported: Hybridization-affinity chromatography of an IVT mixture on a CIMmultus Oligo dT column; equilibration/Wash1 with 50 mM sodium phosphate, 250 mM sodium chloride, 5 mM EDTA, pH 7.0; Wash 2 with 50 mM sodium phosphate, 5 mM EDTA, pH 7.0; elute = 10 mM Tris, pH 8.0.
Hydrophobic-interaction chromatography (HIC) has shown valuable utility for R.N.A. purification. With the correct choice of binding salt, D.N.A. and dsR.N.A. fail to bind. Incomplete transcripts elute before intact ssR.N.A., and NaOH is required to remove the majority of proteins. Reversed-phase chromatography (RPC) using styrenedivinylbenzene media also has proven useful to remove dsR.N.A. and fractionate ssR.N.A. by size. HIC and RPC media are prone to fouling by crude IVT mixtures. They both tolerate extended cleaning with 1 M NaOH, so they can be restored to original condition, but fouling within a run can interfere with purification performance. This makes both of them better suited for polishing.
Figure reported: Hydrophobic interaction chromatography of an IVT mixture on a CIMmultus C4 HLD column; sample loaded in 1.8 M NaCl, pH 7.0, and eluted with a descending salt gradient. The red trace shows a separate dsR.N.A. sample loaded under identical conditions.
R.N.A. Purification by Anion-Exchange Chromatography Ion-exchange chromatography IEC has served protein and plasmid D.N.A. purification for decades. Traditional exchangers such as diethylaminoethyl and quaternary amine work for R.N.A. transcripts smaller than 500 bases , but not for large transcripts. At ambient temperature, their elevated hydrogen bonding capacity prevents them from being eluted with anything less than sodium hydroxide. Heating the buffers and columns into the range of 50–70°C suppresses hydrogen bonding sufficiently to enable elution with sodium chloride gradients.
Heating imposes a burden on process development and manufacturing, but it also provides a clue: An exchanger with less hydrogen bonding capacity should be able to elute R.N.A. at ambient temperature. Figure 10 verifies this prediction by illustrating anion-exchange fractionation IEF of mR.N.A. from plasmid D.N.A. at ambient temperature on a CIMmultus PrimaS column (BIA Separations).
The sample was bound at neutral pH and eluted with an ascending pH gradient. D.N.A. eluted before and well-separated from ssR.N.A.. Double-stranded R.N.A. elutes slightly after D.N.A. but still earlier than ssR.N.A.” (11).
https://bioprocessintl.com/downstream-processing/separation-purification/a-new-runway-for-mR.N.A.-purification/
A New Runway for Purification of Messenger R.N.A.
by Pete Gagnon et al
Thursday, October 29, 2020
Current Tools for Industrial Purification of mR.N.A.
An affinity method for R.N.A. purification already exists. Hybridization-affinity chromatography with an oligo dT (poly-thymidine) ligand captures mR.N.A. by its poly-A tail. About 250 mM sodium chloride suppresses electrostatic repulsion between the negatively charged backbone phosphatidic acid residues on the ligand and on R.N.A. That enables them to approach each other closely. R.N.A. is captured by A-T base pairing. Removing the salt reestablishes their mutual charge repulsion, overwhelms A-T hydrogen bonding, and elutes the R.N.A.
https://bioprocessintl.com/downstream-processing/separation-purification/a-new-runway-for-mR.N.A.-purification/
A New Runway for Purification of Messenger R.N.A.
by Pete Gagnon et al
Thursday, October 29, 2020
Current Tools for Industrial Purification of mR.N.A.
An affinity method for R.N.A. purification already exists. Hybridization-affinity chromatography with an oligo dT (poly-thymidine) ligand captures mR.N.A. by its poly-A tail. About 250 mM sodium chloride suppresses electrostatic repulsion between the negatively charged backbone phosphatidic acid residues on the ligand and on R.N.A. That enables them to approach each other closely. R.N.A. is captured by A-T base pairing. Removing the salt reestablishes their mutual charge repulsion, overwhelms A-T hydrogen bonding, and elutes the R.N.A.
Hybridization-affinity with oligo dT can be used for capture, but it bears some limitations. It cannot discriminate ssR.N.A. from dsR.N.A., and it has no ability to fractionate mR.N.A. according to size. Intact product, incomplete transcripts, oligomers, fragments, aggregates; any species with an accessible poly-A tail elutes with all the rest. Oligo dT can be cleaned with 100 mM NaOH, but higher concentrations are not recommended. This is a concern because of the high fouling potential of IVT mixtures.
Hybridization-affinity chromatography of an IVT mixture on a CIMmultus Oligo dT column; equilibration/Wash1 with 50 mM sodium phosphate, 250 mM sodium chloride, 5 mM EDTA, pH 7.0; Wash2 with 50 mM sodium phosphate, 5 mM EDTA, pH 7.0; elute = 10 mM Tris, pH 8.0.
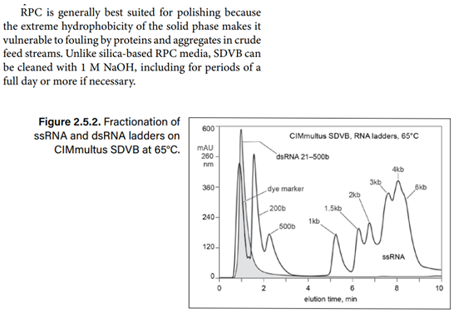
Hydrophobic-interaction chromatography (HIC) has shown valuable utility for R.N.A. purification. With the correct choice of binding salt, D.N.A. and dsR.N.A. fail to bind. Incomplete transcripts elute before intact ssR.N.A., and NaOH is required to remove the majority of proteins. Reversed-phase chromatography (RPC) using styrenedivinylbenzene (SDVB) media also has proven useful to remove dsR.N.A. and fractionate ssR.N.A. by size. HIC and RPC media are prone to fouling by crude IVT mixtures. They both tolerate extended cleaning with 1 M NaOH, so they can be restored to original condition, but fouling within a run can interfere with purification performance. This makes both of them better suited for polishing.
Hydrophobic interaction chromatography HIC of an IVT mixture on a CIMmultus C4 HLD column; sample loaded in 1.8 M NaCl, pH 7.0, and eluted with a descending salt gradient. The red trace shows a separate dsR.N.A. sample loaded under identical conditions.
R.N.A. Purification by Anion-Exchange Chromatography Ion-exchange chromatography IEC has served protein and plasmid D.N.A. purification for decades. Traditional exchangers such as diethylaminoethyl (DEAE) and quaternary amine work for R.N.A. transcripts smaller than 500 bases, but not for large transcripts. At ambient temperature, their elevated hydrogen bonding capacity prevents them from being eluted with anything less than sodium hydroxide . Heating the buffers and columns into the range of 50–70°C suppresses hydrogen bonding sufficiently to enable elution with sodium chloride gradients.
Heating imposes a burden on process development and manufacturing, but it also provides a clue: An exchanger with less hydrogen bonding capacity should be able to elute R.N.A. at ambient temperature. Figure 10 verifies this prediction by illustrating anion-exchange fractionation of mR.N.A. from plasmid D.N.A. at ambient temperature on a CIMmultus PrimaS column (BIA Separations). The sample was bound at neutral pH and eluted with an ascending pH gradient. D.N.A. eluted before and well-separated from ssR.N.A.. Double-stranded R.N.A. elutes slightly after D.N.A. but still earlier than ssR.N.A.
Reversed-phase chromatography of dsR.N.A. and ssR.N.A. ladders on a CIMmultus SDVB column at 65°C; equilibration/wash buffer 0.1 M triethylamino-acetate, pH 7.0; elution buffer 0.1 M triethylaminoacetate, 25% acetonitrile, pH 7.0
Both double-stranded species can be removed instead by a neutral pH wash step with 1M NaCl and 10 mM E.D.TA. Proteins are eliminated with them. Single-stranded R.N.A. remains bound. The column is washed with another buffer to clear the excess salt, then eluted with a pH gradient. Overall purification potential of the pH gradient is increased by eliminating most of the contaminants in advance. That leaves the gradient better able to polish out their last traces. Figure reported shows the results of this approach with the D.N.A. plasmid and ssR.N.A. from the previous figure. Figure reported shows results with dsR.N.A. and ssR.N.A. ladders. Partial size fractionation of ssR.N.A. is evident. The wash step can be intensified by substituting guanidine-hydrochloride for sodium chloride. Combining the chaotropic salt with E.D.T.A simultaneously relaxes nonspecific electrostatic and hydrophobic interactions, hydrogen bonding, and metal coordination. Single-stranded R.N.A. remains bound, which creates an opportunity to scrub and remove contaminants that may have become associated with ssR.N.A. during transcription.
Anion-exchange fractionation AEF of plasmid D.N.A. from ssR.N.A. by pH gradient elution on a CIMmultus PrimaS pH column; Buffer A = 20 mM Tris, 20 mM bis-tris-propane, 20 mM glycine, 50 mM NaCl, 10 mM EDTA, pH 7.0; Buffer B: 20 mM Tris, 20 mM bis-Tris-propane, 20 mM glycine, 50 mM NaCl, 10 mM EDTA, pH 11.0. Nucleic acids are from New England BioLabs.
The pH of the eluted ssR.N.A. should be neutralized as soon as possible after elution. As in the field of protein-affinity chromatography, this is easily done by pre-aliquoting a neutralizing buffer in the fraction vessels or neutralizing ssR.N.A. fractions immediately after completion of the run. Brief exposure to alkaline pH produces no evidence of modification.
Like most anion exchangers, the column can be cleaned for extended periods with 1M NaOH. Exposure to large-volume IVT mixtures generally requires treatment for about an hour. Cleaning can be enhanced by combining 1–3M NaCl and 10–20 mM EDTA with the NaOH. Badly fouled columns can be restored by treatment for 16–24 hours.
Elution of plasmid D.N.A. and ssR.N.A. from a CIMmultus PrimaS column. In one experiment, ssR.N.A. with a size of 5,000 b was applied and eluted in a pH gradient. In the other experiment, plasmid D.N.A. was bound then washed off the column with 1 M NaCl, 10 mM EDTA. Note its absence from the pH gradient except for a small doublet at about 11 minutes. Nucleic acids are from New England BioLabs.
Purification of Research- and Clinical-Grade ssR.N.A. For purification of research-quality ssR.N.A., a CIMmultus PrimaS column with a salt wash before pH elution provides one-step purification performance comparable to what protein A affinity has been providing to immunologists since the 1990s. This gives researchers a simple protocol to obtain small amounts of good quality ssR.N.A. quickly and easily to advance their studies. It gives upstream process developers an easy tool to evaluate the effects of different variables in optimizing their IVT protocols.
Elution of a dsR.N.A. ladder and a ssR.N.A. ladder from a CIMmultus PrimaS column. In one experiment, a ssR.N.A. ladder containing species ranging in size from 200 to 6,000 bases was applied and eluted in a pH gradient. In the other experiment, a dsR.N.A. ladder containing species ranging from 21 to 500 bases was bound, then washed off the column with 1 M NaCl, 10 mM E.D.T.A. Note its absence from the pH gradient. Nucleic acids are from New England BioLabs.
It gives downstream DS developers the high-performing capture foundation needed for purification of clinical-quality ssR.N.A.. It provides high initial purity and removes the foulants that could interfere with polishing methods. Polishing methods that also support removal of dsR.N.A., D.N.A., and proteins and achieve size fractionation suggest themselves as effective partners. The low salt concentration of the ssR.N.A. coming off anion exchange enables smooth transition to RPC. It also provides a smooth workflow with HIC. Just add salt. Both platforms should consistently deliver clinical quality ssR.N.A.
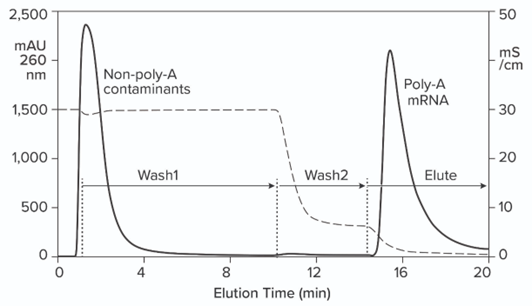
Figure 6: Hybridization-affinity chromatography of an IVT mixture on a CIMmultus Oligo dT column; equilibration/Wash1 with 50 mM sodium phosphate, 250 mM sodium chloride, 5 mM EDTA, pH 7.0; Wash2 with 50 mM sodium phosphate, 5 mM EDTA, pH 7.0; elute = 10 mM Tris, pH 8.0.
Reversed-phase chromatography of dsR.N.A. and ssR.N.A. ladders on a CIMmultus SDVB column at 65°C; equilibration/wash buffer 0.1M triethylaminoacetate, pH 7.0; elution buffer 0.1 M triethylaminoacetate, 25% acetonitrile, pH 7.0
Both double-stranded species can be removed instead by a neutral pH wash step with 1 M NaCl and 10 mM ethylenediaminetetraacetic acid (E.D.T.A). Proteins are eliminated with them. Single-stranded R.N.A. remains bound. The column is washed with another buffer to clear the excess salt, then eluted with a pH gradient. Overall purification potential of the pH gradient is increased by eliminating most of the contaminants in advance. That leaves the gradient better able to polish out their last traces. Partial size fractionation of ssR.N.A. is evident.
Distribution of charged residues, hydrogen donors, and hydrogen acceptors on R.N.A.. Negative charges are indicated in red with yellow halos. Hydrogen acceptors are indicated in red with pairs of red dots to indicate free lone-pairs of electrons. Hydrogen donors are in blue. Compared with the 4 negative charges, there are 11 hydrogen donors and 75 acceptors (one acceptor for each lone pair).
The wash step can be intensified by substituting guanidine-hydrochloride for sodium chloride. Combining the chaotropic salt with E.D.T.A simultaneously relaxes nonspecific electrostatic and hydrophobic interactions, hydrogen bonding, and metal coordination. Single-stranded R.N.A. remains bound, which creates an opportunity to scrub and remove contaminants that may have become associated with ssR.N.A. during transcription.
Anion-exchange AE fractionation of plasmid D.N.A. from ssR.N.A. by pH gradient elution on a CIMmultus PrimaS pH column; Buffer A = 20 mM Tris, 20 mM bis-tris-propane, 20 mM glycine, 50 mM NaCl, 10 mM EDTA, pH 7.0; Buffer B: 20 mM Tris, 20 mM bis-Tris-propane, 20 mM glycine, 50 mM NaCl, 10 mM E.D.T.A, pH 11.0. Nucleic acids are from New England BioLabs.
The pH of the eluted ssR.N.A. should be neutralized as soon as possible after elution. As in the field of protein-affinity chromatography, this is easily done by pre-aliquoting a neutralizing buffer in the fraction vessels or neutralizing the ssR.N.A. fractions immediately after completion of the run. Brief exposure to alkaline pH produces no evidence of modification.
Like most anion exchangers, the column can be cleaned for extended periods with 1 M NaOH. Exposure to large-volume IVT mixtures generally requires treatment for at least an hour. Cleaning can be enhanced by combining 1–3 M NaCl and 10–20 mM E.D.T.A with the NaOH. Badly fouled columns can be restored by treatment for 16–24 hours.”
Majors RE. The Cleaning and Regeneration of Reversed-Phase H.P.L.C. Columns. LC-GC Europe 21(1) July 2003: “Contaminated columns can generate poor peak shapes, nonreproducible retention, high back pressures, and baseline artifacts.Table I lists the most popular stationary
Phases usually bonded to silica gel (1). Phase subspecies — such as mixed phases (in ex, phenyl–hexyl), endcapped and nonendcapped varieties, and polarembedded phases — also exist within these bonded silicas. Various other packing materials have been used in reversed-phase chromatography RPC, including polymers, polymercoated silicas , aluminas, inorganic– organic hybrids, coated zirconia, and graphitized carbon. Each type of phase has its own advantages and disadvantages. Regeneration of Zirconia-Based H.P.L.C. Columns ZirChrom Separations, Inc. ( Minnesota), has manufactured a series of zirconia-based columns. The product line has several reversed-phase columns, including polybutadiene, polystyrene, and graphitized carbon versions.” (6)
J Biomed Biotechnol. 2009; 2009: 574398. D.N.A., R.N.A., and Protein Extraction: The Past and The Present Siun Chee Tan, and Beow Chin Yiap “Nucleic acid purification by using zirconia bead ZB is another type of magnetic bead based purification. These microspherical paramagnetic beads have a large available binding surface and can be dispersed in solution. This characteristic allowed thorough nucleic acid binding, washing, and elution. The total nucleic acid isolation kit, which uses this technology for the nucleic acid purification, makes use of the mechanical disruption of samples with zirconia beads ZB in a guanidinium thiocyanate-based solution that not only releases nucleic acid but also inactivate nuclease in the sample matrix. After the lysis step, dilution of samples is done by using isopropanol. Paramagenetic beads PB are added to the samples for the nucleic acid binding purpose. The mixture of beads and nucleic acid are immobilized on magnets and washed to remove protein and contaminants. Removal of residual binding solution is done with a second wash solution and finally the nucleic acid is eluted in low-salt buffer.” (7)
Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology 1202 2014 Characterisation of Chromatography Media Aimed for Purification of Biomolecules Mikael Andersson “At static conditions a chromatography medium is submitted to harsh (forced) conditions, at extreme pH (HCl pH 1 or NaOH pH 14), high temperature (40°C) and long contact time (one week). Before the treatment, the chromatography medium is pre-treated as described in Papers I and II. It can here be noted that the important step of the pre-treatment part is to remove any carbon-containing compounds in the storage solution of the medium.”
Figure 7: Hybridization-affinity chromatography of an IVT mixture on a CIMmultus Oligo dT column; equilibration/Wash1 with 50 mM sodium phosphate, 250 mM sodium chloride, 5 mM EDTA, pH 7.0; Wash2 with 50 mM sodium phosphate, 5 mM EDTA, pH 7.0; elute = 10 mM Tris, pH 8.0.
Applied Surface Science
Volume 537, 30 January 2021, 148101
Applied Surface Science
Simultaneous growth of graphene/mesoporous silica composites using liquid precursor for H.P.L.C. separations
Hui Qiana Wanying Lia Xuan Wanga Fazhi Xiea W. Lib Qishu Quab
https://doi.org/10.1016/j.apsusc.2020.148101
Volume 537, 30 January 2021, 148101
Applied Surface Science
Simultaneous growth of graphene/mesoporous silica composites using liquid precursor for H.P.L.C. separations
Hui Qiana Wanying Lia Xuan Wanga Fazhi Xiea W. Lib Qishu Quab
https://doi.org/10.1016/j.apsusc.2020.148101
Highlights
- Silica-graphene composites GC were prepared in one step.
- Graphene and silica are simultaneously formed and overlapped to form intercalation composites.
- Ordinary organic solvents are used as carbon sources.
- The preparation process does not need to use graphite as a carbon source.
“Silica intercalated graphene-silica composites (G/SiO2) were prepared by 1-step hydrothermal method. In the previously reported one-step method for synthesizing G/SiO2 composites, graphene needs to be synthesized first and then used to prepare G/SiO2 composites. In this research work, neither the preparation of graphene nor the use of graphite as a carbon source is required for the preparation of G/SiO2 composites. Instead, organic solvent (like toluene, xylene, mesitylene, n-hexane, n-heptane, cyclohexane) was directly used as the carbon source, and the growth and recombination of graphene and SiO2 were achieved simultaneously through a 1-step hydrothermal method. In a hydrothermal reaction vessel, the mixture consisting of organic solvent, TEOS, H2O, and surfactant was uniformly stirred, and then reacted at 160 °C for 4 h to prepare G/SiO2 composite. The influence of the type of organic solvent, hydro-thermal reaction temperature, and calcination temperature on the structure of G/SiO2 composites were studied. The morphology and structure of G/SiO2 composites were characterized by FESEM, T.E.M, and Raman. The formation mechanism of G/SiO2 composite was proposed. G/SiO2 composite was then grown directly on the SiO2 spheres and used as stationary phase SP for high performance liquid chromatograph separation. Mixture of hydrocarbons was well separated under reversed phase condition”. (8)
Turkish Journal of Chemistry
http://journals.tubitak.gov.tr/chem/
Research Article
Extraction of high-purity silica from rice husk via hydrochloric acid leaching treatment
Seitkhan AZAT, Z. SARTOVA, Kalam
07/10/2019
http://journals.tubitak.gov.tr/chem/
Research Article
Extraction of high-purity silica from rice husk via hydrochloric acid leaching treatment
Seitkhan AZAT, Z. SARTOVA, Kalam
07/10/2019
Highly purified amorphous silica samples (98%–99%) with a surface area in the range of 120 to 980 m2g −1 were successfully synthesized using RH from different regions of Kazakhstan. Leaching under HCl pretreatment and controlled calcination at 600°C for 4h revealed a decrease in the metal oxide content in the RH composition.
The purity of synthesized silica was proved by a XRF analysis. The current approach was compared with other studies that also aimed to produce silica samples from RH. The comparison was carried out to show the importance of parameters like as duration and temperature of thermal treatment, rice variety, geographical location, and concentration of used acid. The purpose of acid pre-treatment is to improve the purity and give a high surface area to the silica product during its precipitation. It proves to be an effective way for substantially removing most of the metallic and carbonaceous impurities and producing silica completely white in color. (9)” 2018 JETIR September 2018, Volume 5, Issue 9 Silica Gel, a value added product production from Rice Husk Ash Diksha Srivastava, Nafisa Ali and Dr. Deepak Sharma “On chemical analysis of rice husk ash, it was found that it contains about 80 percent Silica or most commonly known as Silica Gel. Silica Gel is considered as a value added product with commercial sale value of Rs. 200 per Kg. Silica Gel is a non-toxic, non-flammable, non-reactive material. The high surface area of the Silica Gel crystals, allows it to adsorb water easily, thus making it a useful desiccant. Once saturated with water, the gel can be regenerated by heating it to 120°C (250°F) for 2 hours. Other uses of Silica Gel includes its use in Column Chromatography, insulation powder in steel mills and refrigerators, manufacturing of refractory bricks, repellents in the form of ‘vinegar-tar’, etc. With increasing grade quality of Silica Gel, cost also increases. (10)
Silica from Rice as New Drug Delivery Systems Salazar-Hernández Carmen,Salazar-Hernández Mercedes, Lona-Ramos Rocío, Elorza-Rodríguez Enrique and Rocha-Ramírez Agustín Hilario
http://dx.doi.org/10.5772/66723
http://dx.doi.org/10.5772/66723
The pharmaceutical industry has seen an increased need of carriers or excipients design that allows the controlled release of a drug in the human body. The main role of an excipient is to carry the drug for its administration under therapeutic index. Among new generation of excipients, the ordered mesoporous silica (MS) presents several advantages, such as excellent biocompatibility, good adsorption capacity, and precise control in the drug delivery.The high cost of synthesis of mesoporous silica restricts its use to industrial applications; therefore, a low-cost procedure is necessary for the widespread use. Biogenic silica from rice husk (SiO2 -rice) could be a new choice as a drug delivery system. This silica is obtained from an acid leaching of rice husk followed by calcinations processes at low temperatures; these conditions produce a silica with good adsorption properties, similar to those of MS. In consequence, the excipient behavior of SiO2
- Rice was assessed using folic acid as the model drug, displaying 18.5% of absorption in the SiO2
- Rice pores, while MS absorbed around 19%. The drug release profiles were similar for both the silicas, suggesting that SiO2
- Rice could be a low-cost, similar yield excipient for the drugs similar to the folic acid” (11)
Wu, T.; Ke, Q.; Lu, M.;Pan, P.; Zhou, Y.; Gu, Z.; Cui, G.;Lu, H.
Recent Advances in Carbon-Silica Composites:Preparation, Properties, and Applications. Catalysts 2022
Academic Editors: Li Shuai , N. Kumar Gupta
Recent Advances in Carbon-Silica Composites:Preparation, Properties, and Applications. Catalysts 2022
Academic Editors: Li Shuai , N. Kumar Gupta
“Separation is one of the most important unit operations in chemical engineering. In order to prepare a stationary phase with hydrophilic interaction for liquid chromatography, Zhao et al. used silica microspheres as a carrier and cyclodextrin as a carbon source to prepare a carbon-coated composite by hydrothermal carbonization. Cyclodextrin and polyvinylpyrrolidone were first added to a Teflon liner containing deionized water along with the silica microspheres. After the hydrothermal reaction, the slurry-packed capillary columns containing the carbon-silica stationary phase exhibited excellent chromatographic repeatability, separation selectivity, and pH stability for polar compounds, like as phenols and endocrine disrupting chemicals (EDCs;). Since the trade-off between the polarity and selectivity, especially for polar organics, is a common issue in adsorption, Yang et al. Researcher used waste lithium–silicon powder and commercial activated carbon as resources for preparing a zeolite-activated carbon composite material, so as to combine the advantages of activated carbon and molecular sieve”. (12)
Articles
Rice husk derived silica aerogel as chromatographic packing material for colour separation of purple orchid (Cattleya bowringiana) flower
K. N. Maamur ,U. S. Jais
18 Jul 2013
“There are a great number of packing materials commercially available for column chromatography such as silica gel, alumina and cellulose powder. In this study work , silica aerogel prepared using rice husk ash is used as the packing material for colour separation of purple orchid flower. The colour fractions of Cattleya bowringiana extract had been successfully separated using silica aerogel prepared from the rice husk ash by column chromatography. The silica aerogel was prepared by sol–gel and supercritical drying at temperature and pressure of about 250°C and 5·9 MPa (850 psi) respectively. The Cattleya bowringiana extract was applied to the silica aerogel column (BioRad 1 × 20 cm) for separation. Fractionation of the extract was done by elution with 50% aqueous methanol containing 0·05% trifluoroacetic acid. 3 different colours: green, blue and purple were successively parted from the column.”(13)
K. N. Maamur ,U. S. Jais
18 Jul 2013
“There are a great number of packing materials commercially available for column chromatography such as silica gel, alumina and cellulose powder. In this study work , silica aerogel prepared using rice husk ash is used as the packing material for colour separation of purple orchid flower. The colour fractions of Cattleya bowringiana extract had been successfully separated using silica aerogel prepared from the rice husk ash by column chromatography. The silica aerogel was prepared by sol–gel and supercritical drying at temperature and pressure of about 250°C and 5·9 MPa (850 psi) respectively. The Cattleya bowringiana extract was applied to the silica aerogel column (BioRad 1 × 20 cm) for separation. Fractionation of the extract was done by elution with 50% aqueous methanol containing 0·05% trifluoroacetic acid. 3 different colours: green, blue and purple were successively parted from the column.”(13)
Preparation and characterization of amorphous silica and metal modified silica catalyst from rice husk A Thesis Submitted to the National Institute of Technology, Rourkela 2015 “Silica is also used in the extraction of D.N.A. and R.N.A. due to its ability to bind to the nucleic acids under the presence of chaotropes.
Sanhuez has reported the synthesis of value added porous biogenic silica through rice husk ash is better than common water glass. Many researchers have investigated a study on the transformation of amorphous silica to ordered mesoporous MCM-41 or MCM-48 type silica by using the pseudomorphic transformations. An advantage of this method is to produce material with high specific surface area and highly ordered pore structure that can be used as stationary phase in liquid chromatography LC.”(14)
Preparation of SiO2 nanowires from rice husks by hydrothermal method and the R.N.A. purification performance October 2016 Chemical Physics Letters DOI: 10.1016/j.cplett.2016.09.012 Meiyan Huang, J. Cao, Xing Meng, X. Meng et al “In this research study, SiO2 nanowires were prepared by using rice husks as silicon source via a hydrothermal method. The microstructure, thermal stability and morphology of SiO2 nanowires were characterized by X-ray diffraction, infrared spectroscopy, thermal gravimetric analysis and scanning electron microscope. SiO2 nanowires with a diameter of 30–100 nm were obtained and the formation mechanism of SiO2 nanowires during the hydrothermal reaction was proposed. The SiO2 nanowires were introduced into membrane spin columns to isolate R.N.A. and the values of A260/280 and A260/230 were 2.0–2.1 and 1.8–2.0, respectively, which shows the SiO2 nanowires were effective for R.N.A. purification.” (15)
J. Eng. Technol. Sci., Vol. 54, Nov. 18th, 2021. ITB Institute for Research and Community Services Operating Variables on Production of High Purity Bio-silica from Rice Hull Ash by Extraction Process Soen Steven, Elvi Restiawaty , Y. Bindar “Even now, many silica-based products SBP are used for pharmaceutical applications such as drug delivery systems DDS, nanocomposite film for modified-release tablets, and microparticles for promising esophageal mucosal delivery systems . Therefore, proper rice hull utilization is interesting due to its ability to replace fossil resources.
It is economically attractive, has wide applications in terms of energy and chemicals, and has an environmentally friendly production process. Rice hull utilization is usually executed through a calcination phase followed by extraction with an alkaline solvent to produce water glass, also known as the sol-gel method. Subsequently, the water glass is titrated with acid after which the obtained gel begins to precipitate. The precipitate is a colloidal state, which is further aged and dried to obtain bio-silica. The developed sol-gel method has been proven to be capable of synthesizing silica with altered properties like as nano-sized and doped nanocomposites for disinfectant purposes, UV-protective materials , and mesoporous thin film. Rice hull ash extraction to produce high purity bio-silica accompanied by a full factorial design of the experiment was successfully investigated. The effects of 3 variables, like, acid concentration, RS/F, and extraction time, on the bio-silica yield and purity were investigated. Pretreatment with 1 mol/L acid resulted in around 96% of bio-silica purity. Bio-silica yield reaching 97% was also majorly affected by acid concentration, while another main factor that also had a fairly significant effect was RS/F. There was a decreased bio-silica yield of about 8-10% for extraction under RS/F 6 and 2-h extraction time, while simultaneously increasing the acid concentration and RS/F had a synergistic effect on the bio-silica yield. This research study confirmed that high purity bio-silica with a particle size of 5-20 microns was successfully produced from rice hull ash. Variable screening was able to cut the industrial production cost and time by half.” (16)
SK S. Hossain, Lakshya Mathur & P.K. Roy (2018) Rice husk/rice husk ash as an alternative source of silica in ceramics: A review, Journal of Asian Ceramic Societies “High-purity nano-silica HPNS has recently found wide-ranging applications in different fields such as pharmaceuticals, dyes, chromatography, drug delivery systems DDS, electronic components, catalysts and adsorbent materials. As a result, demand for highpurity silica is increasing. Combustion-derived R.H.A. (without acid or alkali treatment) contain less than 95 wt.% of SiO2, and the remaining part comprises different alkali oxides and impurities. With appropriate acid or alkali treatment of RH/R.H.A., the SiO2 content can be increased in the system to more than 99 wt.%. Several researchers have therefore adopted different chemical routes to derive high-purity nano-silica.” (17)
Articles Mat. Res. 20 (Suppl 2) 2017 Characterization of Silica Produced from Rice Husk Ash: Comparison of Purification and Processing Methods Iara Janaína Fernandes et al
“The scientific literature review showed that most techniques include a pretreatment like acid or alkaline leaching followed by thermal treatment to increase the amount of silica produced by reduction of carbonaceous materials. The results showed that it is possible to produce silica from R.H.A. using simple methods, and that these produced silica with purity above 98%. The treatments that afforded the best results were acid leaching followed by thermal treatment at 800ºC, and alkaline extraction at low temperature, with silica purity of 99.3% and 99.6%, respectively.The results of the present study work show that it is possible to produce silica from R.H.A. using simple processes. The various production methods tested afforded to obtain silica with purity above 98%, especially acid leaching followed by thermal treatment and sol-gel alkaline extraction at low temperature, when silica contents were between 99.3% - 99.6%, respectively. Alkaline extraction at low temperature also produced silica particles with large specific surface area (about 290 m²/g), compared with the other treatments evaluated, and proving to be the most advantageous technique.
The purity values observed, between 98.7%-99.6%, are high, compared with data reported in the literature, especially considering the fact that most studies extracted silica directly from unburnt rice husk. Ugheoke and Mamat19 explain that one of the reasons why it is more difficult to obtain silica with purity above 97% from rice husk by direct incineration is the presence of metallic contaminants, especially potassium and sodium oxides. These compounds affect the surface of silica particles, increasing their surface area SA and reactivity, and increase silica crystallization rate CR. Acid leaching followed by thermal treatment (LX TT 800 1h) induced the production of silica with the lowest carbon level (0.09%). Krishnarao et al. Researcher explained that the acid leaching prevents the formation of black particles, since it removes potassium, the main agent responsible for the presence of unburnt carbon in ash. The authors showed that potassium works as catalyst in the crystallization of silica and, when temperature rises above the dissociation temperature of K2O (approximately 347ºC), the surface of ash particles begins to melt, blocking the transportation of oxygen and CO2 and increasing the amount of the unburnt carbon. The low potassium level (0.48%) found in this sample may explain the smaller amount of carbon. “(18)
Preparation and Characterization of Bioactive Silica-based Ceramics derived from Rice Husk Ash A Thesis Submitted in Partial Fulfillment of the Requirements for the Degree of
DOCTOR OF PHILOSOPHY
By Jyoti Prakash Nayak
DEPARTMENT OF CERAMIC ENGINEERING
NATIONAL INSTITUTE OF TECHNOLOGY, ROURKELA
AUGUST, 2010
DOCTOR OF PHILOSOPHY
By Jyoti Prakash Nayak
DEPARTMENT OF CERAMIC ENGINEERING
NATIONAL INSTITUTE OF TECHNOLOGY, ROURKELA
AUGUST, 2010
“Amorphous silica of R.H.A. has been widely used in vegetable oil refining, pharmaceutical products, detergents, adhesives, chromatograph column packing, and different ceramic compositions.”(19)
Reference Module in Materials Science and Materials Engineering 2016 Monolithic Materials for Bio-Separations R.J.Groarke,D.Brabazon “Monolithic materials have been investigated as candidates for the efficient separation of chemical and biological species for many years. This research article discusses the advances in this area with particular emphasis on poly-methacrylate and silica monoliths, and on the use of these in the separation and enrichment of biomolecules, such as D.N.A., proteins, amino acids and plasmids. A brief history of monoliths is given, followed by a more detailed discussions on the preparation methods and applications of methacrylate and silica-based monoliths that have been reported. Other monolithic materials like as carbon and nanoparticle-functionalized monoliths are also described “(20) Talanta. 2014 Nov Facile preparation of octadecyl monoliths with incorporated carbon nanotubes and neutral monoliths with coated carbon nanotubes stationary phases for H.P.L.C. of small and large molecules by hydrophobic and π-π interactions.
Erandi Mayadunne, Z. El Rassi “2 approaches for incorporating carbon nanotubes into monolithic columns for H.P.L.C. are described in this report. They pertain to the investigation of carbon nanotubes either (i) as entities to modulate solute retention on monolithic columns bearing well defined retentive ligands or (ii) as entities that constitute the stationary phase responsible for solute retention and separation. Approach (i) involved the incorporation of carbon nanotubes into octadecyl monolithic columns while approach (ii) concerns the preparation and evaluation of an ideal monolithic support and coating it with carbon nanotubes to yield a real "carbon nanotube stationary phase" for the H.P.L.C separation of a wide range of solutes. First of all , an octadecyl monolithic column based on the in situ polymerization of octadecyl acrylate and trimethylolpropane trimethacrylate was optimized for use in H.P.L.C. separations of small - large solutes (like, proteins). To further modulate the retention and separation of the proteins, small amounts of carbon nanotubes were incorporated into the octadecyl monolith column. In approach (ii), an inert, relatively polar monolith based on the in situ polymerization of glyceryl monomethacrylate and ethylene glycol dimethacrylate (EDMA) proved to be the most suitable support for the preparation of "carbon nanotube stationary phase". This carbon nanotube "coated" monolith proved useful in the H.P.L.C. separation of a wide range of small solutes including enantiomers. In approach (ii), a more homogeneous incorporation of carbon nanotubes into diol monolithic columns ( GMM/EDMA) was achieved when hydroxyl functionalized carbon nanotubes were incorporated into the GMM/EDMA monolithic support. High power sonication for a short time enhanced further the homogeneity of the monolith incorporated with the nanotubes. In all cases, nonpolar and π interactions were responsible for solute retention on the monolith incorporated carbon nanotubes.” (21)
From https://www.science.org/cms/asset/ae501916-0601-46d0-828a-21adad7e9522/productads.pdf
“6 new offerings have been added to the Onyx monolithic high-performance liquid chromatography (H.P.L.C.) column line, including a 150 x 0.05 mm ID choice for proteomics research. Optimized for the low flow rates commonly used for liquid chromatography/mass spectrometry LC/MS proteomics analysis, this column delivers high sensitivity for the biomarker discovery. Other new column sizes include a 0.2 mm C18 column for higher flow rates, a 150 x 0.2 mm trapping column, a 150 x 0.1 mm C8 column, and 2 ultrahigh-resolution C18 columns at 100 micron and 200 micron IDs. The permeable Onyx media separates viscous biological samples that clog traditional columns.
These columns contain solid rods of fused silica and eliminate packing issues associated with particulate media. Run times are as much as 50% faster than with traditional columns.” 2014 PURIFICATION AND EFFECTIVENESS OF VACCINES AND ANTIVIRAL COMPOUNDS
Maria F. Gencoglu Michigan Technological University “Chromatography is the most prevalent technology for large-scale purification of viral particles, since it is fast, scalable and reproducible” (22)
Purification of Recombinant Vaccinia Virus for Oncolytic and Immunotherapeutic Applications using Monolithic Column Technology David Isaac William Vincent “Other manufacturers of monoliths are BioRad who have produced polyacrylamide UNO columns. These are advertised for their high resolution at high flow rates for protein separation. Merck have produced a silica-based monolith called Chromolith, but have concentrated on the analytical ability of the columns taking advantage of their high number of theoretical plates compared to the packed bead resins of an equivalent bed length”.
From Chromolith® High Resolution H.P.L.C. Columns by Merck KGaA, Darmstadt, Germany “Chromolith® HighResolution: High resolution separations without ultra high pressure. The revolutionary monolithic Chromolith® HighResolution columns are made from a single rod of high-purity monolithic silica, rather than from particles.
Their unique bimodal pore structure allows the chromatographic performance to be improved significantly.
Speed of analysis (separations up to 9 times faster if required)
Separations 2 times faster at half the column back-pressure compared to μm columns
Higher sample throughput
Improved H.P.L.C. system security
Significantly increased column lifetime
Reduced maintenance on H.P.L.C. pump and injector seals
Reduced need for sample preparation as columns are very resistant to blocking (even with biological samples)
Column length no longer pressure limited
Very high separation efficiency by column coupling”
Separations 2 times faster at half the column back-pressure compared to μm columns
Higher sample throughput
Improved H.P.L.C. system security
Significantly increased column lifetime
Reduced maintenance on H.P.L.C. pump and injector seals
Reduced need for sample preparation as columns are very resistant to blocking (even with biological samples)
Column length no longer pressure limited
Very high separation efficiency by column coupling”
Google patent
WO2006132588A1 WIPO (PCT)
Method for the purification of synthetic oligonucleotides containing one or several labels
WO2006132588A1 WIPO (PCT)
Method for the purification of synthetic oligonucleotides containing one or several labels
“Analytical liquid chromatography of the purified labeled oligonucleotides was performed on an Agilent H.P.L.C. system, equipped with a Chromolith”
December 2019 Development of F elopment of Functionaliz unctionalized Capillar ed Capillary-Channeled P y-Channeled Polymer (C- olymer (CCP) Fibers as Stationary Phase for Affinity Chromatography
Hung Khiem Trang Clemson University “Different approaches for the immobilizing affinity ligands onto stationary phases include non-covalent methods like non-specific and bio-specific adsorption, covalent coupling techniques, entrapment, and molecular imprinting. Non-specific adsorption of ligands onto support is among the earliest ligand immobilization methods reported. This technique relies purely on the physical adsorption of the ligand to a support through the intermolecular forces including the Coulombic interactions, hydrogen bonding, and hydrophobic interactions . Due to its simplicity and convenience, nonspecific immobilization has been still commonly used and applied to various supports including alumina, silica, carbon or charcoal, collagen and metals ”
From Merck website Race through your Separations on Any System Chromolith® Monolithic Silica H.P.L.C. Column “Chromolith® monolithic silica columns provide an excellent solution for the separation of pharmaceutical compounds, including those pharmaceuticals analyzed by pharmacopeia methods”.
Anal Bioanal Chem. 2013 Mar High-resolution monolithic columns--a new tool for effective and quick separation Hana Sklená?ová et al “This work describes a comparison of 3 types of commercial high-performance liquid chromatography silica monolithic columns with different inner diameters and generations of monolithic sorbent: a "classic" monolithic column, the first generation (Onyx™ monolithic C(18), 100 mm × 4.6 mm, Phenomenex); a "narrow" monolithic column for fast separation at lower flow rates (Chromolith® Performance RP-18e, 100 mm × 3 mm, Merck); and a recently introduced "high-resolution" monolithic column, the next generation (Chromolith® HighResolution RP-18e, 100 mm × 4.6 mm, Merck).” (23)
Experimental Project Hypotesys
In order to verify the role played by the silica gel for chromatografic separation and purification of m R.N.A.
In order to verify the role played by the silica gel for chromatografic separation and purification of m R.N.A.
It is necessary to test 3 group of sample: puridification of m R.N.A.
- Using chromatographic resin silica based of sysntetic origin
- Using resin based on rice production ( high purity like 99%) and with low carbon content
- Using the same resin from rice but with not high purity like 94% and relevant carbon content
It is needed to test the purified product for carbon compound – graphene. (chemico- analytical test) the results if show significative difference (p > 0,05) indicate a role played by the use of silica from Rice.
Discussion
In laste decades various purification methods was and are used to purify m R.N.A. In research and testing field are currently used magnetic beads also covered with graphene or carbon dots. The same are used membrane or chromatographic tecnique (resin)
Today are also used monoliths with composite materials. The process used for purification reported in literature include various phases: TFF, affinty od ion exchange chromatograpy, followed by ultrafiltration/diafiltration. The material used are often : plastic material for TFF filtration, silica products or other polymer. For ultrafiltration and diafiltration various materials for the membrane are used. Of interest that a great producer of monoliths use carbon fibre reinforcement embedded into epoxy thermoset resin (carbon fibre composite); Related silica colum and resin it is possible to see that there is an pharmaceutical use and the syntetic one meet this quality property as required by the pharmacopeia better the from rice. But the same some producer provide this silica product from Rice as pharma eccipient.
There are also works that show that the production form rice can respect the quality required by a pharmacopeia. This silica from rice vary in the purity from in example 80 -99 % and show impuirty ( also carbon product in example 1% o more)
The cost of this 2 kind of product can explain the motivation to use or not a product. in the website of SIGMA ADLRICH is reported this reference for silica gel production for chromatography https://www.sigmaaldrich.com/IT/it/product/sial/60736 A simple method for production of pure silica from rice hull ash Kalapathy, U., A. Proctor, and J. Shultz. Bioresource Technology, 73, 257-262 (2000)
Conclusion
Related the use of graphene derivates in biopharmaceutical it is of of interest the fact that: in a google patent WO2014152031A1 (cited by MERCK website): https://www.sigmaaldrich.com/IT/en/technical-documents/technical-article/pharmaceutical-and-biopharmaceutical-manufacturing/vaccine-manufacturing/manufacturing-strategies-for-mR.N.A.-vaccines
Ribonucleic acid purification : related resin : Exemplary materials that can be used as a surface include, but are not limited to acrylics, carbon ( graphite, carbon-fiber). But also the role played by silica in purification of the m R.N.A.. ( resins, monolits ) or by other polymeric molecule.
A great producer report into its products monolits based on carbon composite material for purifying
R.N.A. (Carbon fibre reinforcement embedded into epoxy thermoset resin).
Of great interest also to verify if the origin of this silica if used and if carbon coated or not.
If of sysntetic origin or form natural product ( form Rice or algae).
Because due by the production process are used also real high temperature a carbon content can be finded in this resin.
The silica from rice are also used in pharmaceuticals as eccipients , and literature report way to produce in high % ( 94-99%) with variable concentration of impurities (1% or more).
On the website of SIGMA ADLRICH is reported an interesting reference related silica gel production for chromatography https://www.sigmaaldrich.com/IT/it/product/sial/60736
A simple method for production of pure silica from rice hull ash Kalapathy, U., A. Proctor, and J. Shultz.
Bioresource Technology, 73, 257-262 (2000)
After seeing all reported in this work it is relevant to verify :
A great producer report into its products monolits based on carbon composite material for purifying
R.N.A. (Carbon fibre reinforcement embedded into epoxy thermoset resin).
Of great interest also to verify if the origin of this silica if used and if carbon coated or not.
If of sysntetic origin or form natural product ( form Rice or algae).
Because due by the production process are used also real high temperature a carbon content can be finded in this resin.
The silica from rice are also used in pharmaceuticals as eccipients , and literature report way to produce in high % ( 94-99%) with variable concentration of impurities (1% or more).
On the website of SIGMA ADLRICH is reported an interesting reference related silica gel production for chromatography https://www.sigmaaldrich.com/IT/it/product/sial/60736
A simple method for production of pure silica from rice hull ash Kalapathy, U., A. Proctor, and J. Shultz.
Bioresource Technology, 73, 257-262 (2000)
After seeing all reported in this work it is relevant to verify :
- If are used in purification of m R.N.A. for vaccine magnetic beads or resin graphene coated or monoliths of carbon composite materials
- What kind of silica is used ( syntetic or form rice or other natutal product)
- The level of graphene impurity in this chromatographic stationary phases.
All this make possible to verify the role played by this phenomena in the profile of impurity in biopharmaceutical production.
This is relevant for regulatory , toxicological and pathological and safety need
This is relevant for regulatory , toxicological and pathological and safety need
Conflic of Interest: NO
References
- SK S. Hossain, Lakshya Mathur & P.K. Roy. (2018). Rice husk/rice husk ash as an alternative source of silica in ceramics: A review, Journal of Asian Ceramic Societies, 6:4, 299-313.
- MOLECULES Review Silica-Based Monolithic Columns as a Tool in H.P.L.C.—An Overview of Application in Analysis of Active Compounds in Biological Samples Micha? Staniak, Magdalena Wójciak, Ireneusz Sowa, atarzyna Tyszczuk-Rotko, Maciej Strzemski, S?awomir Dresler and Wojciech My?li?ski (2020).
- Kumar, Anil et al. (2018). “ESTABLISHMENT OF RICE HUSK BY-PRODUCT AS PHARMACEUTICAL EXCIPIENTS.”.
- Silica from Rice as New Drug Delivery Systems March 2017 DOI: 10.5772/66723 In book: Rice - Technology and Production Carmen Salazar-Hernández Mercedes Salazar Mercedes Salazar Rocío Lona-Ramos Show et al
- Majors RE. (2003). The Cleaning and Regeneration of Reversed-Phase H.P.L.C. Columns. LC-GC Europe 21(1) July: 19–26.
- J Biomed Biotechnol. (2009). D.N.A., R.N.A., and Protein Extraction: The Past and The Present Siun Chee Tan, and Beow Chin Yiap
- Applied Surface Science Volume 537, 30 January (2021). Applied Surface Science Simultaneous growth of graphene/mesoporous silica composites using liquid precursor for H.P.L.C. separations Hui Qiana Wanying Lia Xuan Wanga Fazhi Xiea Weihua Lib Qishu Quab
- Turkish Journal of Chemistry http://journals.tubitak.gov.tr/chem/ Research Article Extraction of high-purity silica from rice husk via hydrochloric acid leaching Treatment Seitkhan AZAT, Zhanar SARTOVA, Kalam0 7/10/2019
- JETIR September (2018). Volume 5, Issue 9 Silica Gel, a value added product production from Rice Husk Ash Diksha Srivastava, Nafisa Ali and Dr. Deepak Sharma
- Silica from Rice as New Drug Delivery Systems Salazar-Hernández Carmen,Salazar-Hernández Mercedes, Lona-Ramos Rocío,Elorza-Rodríguez Enrique and Rocha-Ramírez Agustín Hilario
- Wu, T.; Ke, Q.; Lu, M.;Pan, P.; Zhou, Y.; Gu, Z.; Cui, G.;Lu, H. Recent Advances in Carbon-Silica Composites: Preparation, Properties, and Applications. Catalysts (2022). Academic Editors: Li Shuai and Navneet Kumar Gupta
- Rice husk derived silica aerogel as chromatographic packing material for colour separation of purple orchid (Cattleya bowringiana) flower K. N. Maamur &U. S. Jais 18 Jul (2013).
- Preparation and characterization of amorphous silica and metal modified silica catalyst from rice husk A Thesis Submitted to the National Institute of Technology, Rourkela (2015)
- Preparation of SiO2 nanowires from rice husks by hydrothermal method and the R.N.A. purification performance October (2016). Chemical Physics Letters. Meiyan Huang Jianping Cao Xing Meng Xing Meng et al
- J. Eng. Technol. Sci., Vol. 54, No. 3, (2022). ITB Institute for Research and Community Services. Operating Variables on Production of High Purity Bio-silica from Rice Hull Ash by Extraction Process Soen Steven, Elvi Restiawaty & Yazid Bindar
- SK S. Hossain, Lakshya Mathur & P.K. Roy (2018). Rice husk/rice husk ash as an alternative source of silica in ceramics: A review, Journal of Asian Ceramic Societies, 6:4, 299-313.
- Mat. Res. 20 (Suppl 2) (2017). Characterization of Silica Produced from Rice Husk Ash: Comparison of Purification and Processing Methods Iara Janaína Fernandes Daiane Calheiro Felipe A. L. Sánchez Alini Luísa Diehl Camacho Tatiana Louise Avila de Campos Rocha Carlos Alberto Mendes Moraes Vânia Caldas de Sousa
- Preparation and Characterization of Bioactive Silica-based Ceramics derived from Rice Husk Ash A Thesis Submitted in Partial Fulfillment of the Requirements for the Degree of DOCTOR OF PHILOSOPHYBy Jyoti Prakash Nayak DEPARTMENT OF CERAMIC ENGINEERING NATIONAL INSTITUTE OF TECHNOLOGY, ROURKELA August , 2010
- Reference Module in Materials Science and Materials Engineering (2016) Monolithic Materials for Bio-Separations R.J.GroarkeD.Brabazon
- Talanta. (2014). Facile preparation of octadecyl monoliths with incorporated carbon nanotubes and neutral monoliths with coated carbon nanotubes stationary phases for H.P.L.C. of small and large molecules by hydrophobic and π-π interactions Erandi Mayadunne , Ziad El Rassi
- Maria F. Gencoglu Michigan Technological University. (2014). PURIFICATION AND EFFECTIVENESS OF VACCINES AND ANTIVIRAL COMPOUNDS.
- Anal Bioanal Chem. (2013). High-resolution monolithic columns--a new tool for effective and quick separation Hana Sklená?ová 1, Petr Chocholouš, Petra Koblová, Lukáš Zahálka, Dalibor Šatínský, Ludmila Matysová, Petr Solich.
Citation: Mauro Luisetto, Nili B. Ahmadabadi, Zaineb O. Ettarhouni, Edbey K and Latyshev OY. (2022). Monoliths in the mR.N.A. Vaccine Purification Process the Silica Resin and other Composite Materials : the Carbon Content. Journal of Pharmacy and Drug Development 4(1).
Copyright: © 2022 Mauro Luisetto. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.