Review Article
Volume 7 Issue 1 - 2025
A Systematic Review of Prophylactic Corticosteroids in the Prevention of Fat Embolism Syndrome Following Long Bone Fractures
1MD, MRCS, MSc. Department of Orthopaedics, Trauma and Sports Medicine, Mater Dei Hospital, Msida Malta
2BSc (Hons), MD, MRCPsych, North London NHS Foundation trust, United Kingdom
3MBCHB, FRACS, FRCS. Department of Orthopaedics, Queen Mary University of London, United Kingdom
2BSc (Hons), MD, MRCPsych, North London NHS Foundation trust, United Kingdom
3MBCHB, FRACS, FRCS. Department of Orthopaedics, Queen Mary University of London, United Kingdom
*Corresponding Author: Luca Calleja, MD, MRCS, MSc. Department of Orthopaedics, Trauma and Sports Medicine, Mater Dei Hospital, Msida Malta.
Received: August 04, 2025; Published: August 12, 2025
Abstract
Background: Fat embolism syndrome (FES) is a life-threatening condition that occurs in patients who have experienced severe trauma and/or long bone fractures. Apart from supportive care, some small-scale clinical trials have indicated potential benefits of using corticosteroids in preventing FES; however, remains a topic of debate.
Aims: The primary aim was to evaluate the effect of prophylactic corticosteroids in preventing FES in patients with long bone fractures. Secondary aims evaluated the effects on hypoxaemia, mortality, risks of infection, and avascular necrosis.
Methods: A systematic search was performed using PubMed, EMBASE, and others, for studies that used prophylactic corticosteroids in patients with at least one long bone fracture.
Results: Out of 112 studies, 8 met our eligibility criteria, assessing 545 patients. A total of 246 patients received Methylprednisolone, while 299 patients formed the control group. 10 patients within the steroid receiving groups and 59 patients within the control groups developed FES (P < 0.05). Similarly, corticosteroids also reduced the risk of hypoxia.
Conclusion: The use of corticosteroids may have a positive effect in preventing FES and hypoxia in patients with long bone fractures. Trials conducted so far have certain limitations. Therefore, a large-scale randomized trial is needed for further confirmation and validation of these findings.
Keywords: Fat Embolism Syndrome; FES; Corticosteroids; Methylprednisolone; Long bone fracture
Abbreviations: Fat Embolism Syndrome: FES; Acute respiratory distress syndrome: ARDS; Randomised controlled trails: RCTs
Introduction
Fat embolism syndrome (FES) is commonly observed in patients with multiple long-bone fractures, particularly those who have experienced significant trauma. FES is a serious life-threatening condition [1,2]. It primarily affects individuals in their twenties or thirties, which aligns with the typical trauma demographic. Studies have reported the incidence of FES after trauma to be up to ten percent, with evidence of fat emboli observed in up to nine out of ten patients with long-bone fractures [3,4] FES can also occur during orthopaedic procedures that entail intramedullary reaming [5]. The mortality rate associated with FES is believed to range from 10% to 20% [3,4].
The diagnosis of FES relies on three clinical observations encompassing respiratory, neurological, and cutaneous symptoms [6,7]. The treatment approach for FES revolves around supportive care and early fixation of fractures [8,9]. There is ongoing debate regarding the pharmacological treatment of FES, despite previous utilization of various agents aside from corticosteroids [10]. FES is thought to develop due to the release of free fatty acids during triglyceride breakdown, which damages the lung tissue through vasculitis and endothelial damage [11]. Corticosteroids have been suggested as a means to limit the increase in free fatty acid levels and mitigate the inflammation. Although small trials have demonstrated the beneficial effects of corticosteroids in preventing FES, their use in trauma patients have been limited due to concerns about infection and potential mortality. Lately, the administration of "low-dose" corticosteroids has shown substantial benefits for patients in the early stages of acute respiratory distress syndrome (ARDS) [12]. Consequently, it remains uncertain whether corticosteroids can effectively prevent the FES from occurring in multiple long-bone fractures patients.
Primary and Secondary Outcomes
The primary aim of this study was to evaluate the effect of prophylactic corticosteroids in preventing the development of Fat Embolism Syndrome (FES) when given to patients with long bone fractures.
The primary aim of this study was to evaluate the effect of prophylactic corticosteroids in preventing the development of Fat Embolism Syndrome (FES) when given to patients with long bone fractures.
The secondary aims of this study were to evaluate the development of hypoxaemia and whether corticosteroids could be administered safely by assessing any complications associated with them, such as mortality, increased risks of infection, and avascular necrosis.
Introduction
Fat embolism syndrome (FES) is commonly observed in patients with multiple long-bone fractures, particularly those who have experienced significant trauma. FES is a serious life-threatening condition [1,2]. It primarily affects individuals in their twenties or thirties, which aligns with the typical trauma demographic. Studies have reported the incidence of FES after trauma to be up to ten percent, with evidence of fat emboli observed in up to nine out of ten patients with long-bone fractures [3,4] FES can also occur during orthopaedic procedures that entail intramedullary reaming [5]. The mortality rate associated with FES is believed to range from 10% to 20% [3,4].
The diagnosis of FES relies on three clinical observations encompassing respiratory, neurological, and cutaneous symptoms [6,7]. The treatment approach for FES revolves around supportive care and early fixation of fractures [8,9]. There is ongoing debate regarding the pharmacological treatment of FES, despite previous utilization of various agents aside from corticosteroids [10]. FES is thought to develop due to the release of free fatty acids during triglyceride breakdown, which damages the lung tissue through vasculitis and endothelial damage [11]. Corticosteroids have been suggested as a means to limit the increase in free fatty acid levels and mitigate the inflammation. Although small trials have demonstrated the beneficial effects of corticosteroids in preventing FES, their use in trauma patients have been limited due to concerns about infection and potential mortality. Lately, the administration of "low-dose" corticosteroids has shown substantial benefits for patients in the early stages of acute respiratory distress syndrome (ARDS) [12]. Consequently, it remains uncertain whether corticosteroids can effectively prevent the FES from occurring in multiple long-bone fractures patients.
Primary and Secondary Outcomes
The primary aim of this study was to evaluate the effect of prophylactic corticosteroids in preventing the development of Fat Embolism Syndrome (FES) when given to patients with long bone fractures.
The primary aim of this study was to evaluate the effect of prophylactic corticosteroids in preventing the development of Fat Embolism Syndrome (FES) when given to patients with long bone fractures.
The secondary aims of this study were to evaluate the development of hypoxaemia and whether corticosteroids could be administered safely by assessing any complications associated with them, such as mortality, increased risks of infection, and avascular necrosis.
Materials and Methods or Experimental Procedures
Review Registration
The systematic review was registered with the International Prospective Register of Systematic Reviews (PROSPERO) under the ID number CRD42023392311.
The systematic review was registered with the International Prospective Register of Systematic Reviews (PROSPERO) under the ID number CRD42023392311.
Data Sources and Search Strategy
A systematic search was performed between the months of December 2022 and February 2023 using the following electronic databases; PubMed, EMBASE, SCOPUS and the Cochrane Central Register of Controlled Trials. The search strategy was devised using the PICO format. The search utilized keywords and free-text terms, in conjunction with Boolean operators and MeSH terms. For this systematic review, the most common keywords used were ‘corticosteroid*’, ‘methylprednisolone’, ‘steroid*’, ‘fat embolism syndrome’ and ‘fracture*’. Other potential studies were screened by utilizing the articles’ bibliographies. The risk of biased decisions was reduced by using a second reviewer who assessed the studies and agreed with the search strategy, and hence the selection of the final studies to be included.
A systematic search was performed between the months of December 2022 and February 2023 using the following electronic databases; PubMed, EMBASE, SCOPUS and the Cochrane Central Register of Controlled Trials. The search strategy was devised using the PICO format. The search utilized keywords and free-text terms, in conjunction with Boolean operators and MeSH terms. For this systematic review, the most common keywords used were ‘corticosteroid*’, ‘methylprednisolone’, ‘steroid*’, ‘fat embolism syndrome’ and ‘fracture*’. Other potential studies were screened by utilizing the articles’ bibliographies. The risk of biased decisions was reduced by using a second reviewer who assessed the studies and agreed with the search strategy, and hence the selection of the final studies to be included.
Eligibility Criteria
The search for this systematic review was restricted to full text articles written in English, with no publication date limit. It was also limited to only RCTs and qRCTs of patients with at least one long bone fracture. Studies that didn’t specify what type of fracture was present and studies that included patients with multiple traumas with injuries to the head, thorax and abdomen were excluded. We recognized that the diagnosis of FES is that of a clinical analysis which has varied over the years; therefore, we decided to depend on the authors’ individual criteria for diagnosis. The studies included in this systematic review in accordance with the eligibility criteria are listed below in Table 1.
The search for this systematic review was restricted to full text articles written in English, with no publication date limit. It was also limited to only RCTs and qRCTs of patients with at least one long bone fracture. Studies that didn’t specify what type of fracture was present and studies that included patients with multiple traumas with injuries to the head, thorax and abdomen were excluded. We recognized that the diagnosis of FES is that of a clinical analysis which has varied over the years; therefore, we decided to depend on the authors’ individual criteria for diagnosis. The studies included in this systematic review in accordance with the eligibility criteria are listed below in Table 1.
| PICO | Inclusion Criteria | Exclusion Criteria |
| Population | Patients 16 yrs or older with at least one long bone fracture | Patients with multiple traumas and/or undergoing orthopaedic intervention |
| Intervention | Administration of prophylactic corticosteroids | Administration of therapeutic corticosteroids |
| Comparison | Compared Steroids to a placebo or did not administer | Studies not having a control |
| Outcome | The incidence of development of FES when given steroids prophylactically | Studies that did not examine FES development, but only hypoxaemia |
Table 1: Inclusion and exclusion criteria.
Data extraction and Management
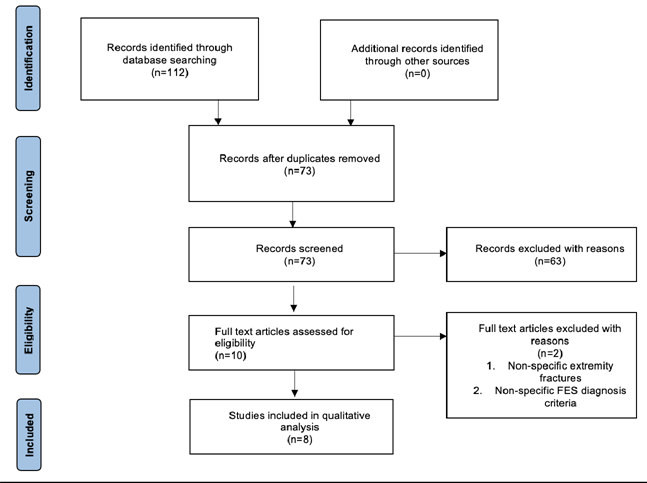
Two reviewers independently screened all the article titles, abstracts and evaluated their eligibility according to the table of inclusion and exclusion criteria demonstrated in Table 1. Disagreements were settled by using a third reviewer. The studies to be evaluated for inclusion/exclusion were listed in a custom-made table on Microsoft Excel. This table highlights the PICO components of each study, allowing screening to be more accurate and efficient. If a decision could not be taken whether to include a study from only its title or abstract, the full text was retrieved for further evaluation. When full texts were not available, and the study was to be included or potentially included after further analysis of the full text, the authors or library were contacted for potential retrieval of the full text. The stages of the data collection process are outlined in the PRISMA [13]. Flow diagram seen in Figure 1. The studies that were selected for this systematic review can be seen in Table 2, where the study characteristics are outlined enabling comparison of their PICO components.
Two reviewers independently screened all the article titles, abstracts and evaluated their eligibility according to the table of inclusion and exclusion criteria demonstrated in Table 1. Disagreements were settled by using a third reviewer. The studies to be evaluated for inclusion/exclusion were listed in a custom-made table on Microsoft Excel. This table highlights the PICO components of each study, allowing screening to be more accurate and efficient. If a decision could not be taken whether to include a study from only its title or abstract, the full text was retrieved for further evaluation. When full texts were not available, and the study was to be included or potentially included after further analysis of the full text, the authors or library were contacted for potential retrieval of the full text. The stages of the data collection process are outlined in the PRISMA [13]. Flow diagram seen in Figure 1. The studies that were selected for this systematic review can be seen in Table 2, where the study characteristics are outlined enabling comparison of their PICO components.
Data Analysis
The data obtained from the studies included in the analysis showed significant diversity. Authors utilized different criteria for diagnosing FES, with some creating scoring methods. Although all studies used Methyl prednisolone, each study was unique in terms of dosage and frequency/duration. Control groups also varied, with some receiving placebos such as saline or dextrose, while other studies gave nothing to patients in the control groups. Population sizes, albeit small, also varied. As a result, it was not suitable for a meta-analysis, and instead, a narrative analysis was conducted. The assessment of the quality of the studies included in the analysis followed the Critical Appraisal Skills Programme (CASP) Tool guidelines for both RCTs and qRCTs [14].
The data obtained from the studies included in the analysis showed significant diversity. Authors utilized different criteria for diagnosing FES, with some creating scoring methods. Although all studies used Methyl prednisolone, each study was unique in terms of dosage and frequency/duration. Control groups also varied, with some receiving placebos such as saline or dextrose, while other studies gave nothing to patients in the control groups. Population sizes, albeit small, also varied. As a result, it was not suitable for a meta-analysis, and instead, a narrative analysis was conducted. The assessment of the quality of the studies included in the analysis followed the Critical Appraisal Skills Programme (CASP) Tool guidelines for both RCTs and qRCTs [14].
Quality Appraisal, Risk of bias and Publication bias assessment
Following the recommendations of the Cochrane Handbook of Systematic Reviews for interventions, we assessed the risk of bias for RCTs using the Cochrane risk-of-bias tool for randomised trials (RoB2) and the ROBINS-I tool was used to assess for bias in qRCTs [15,16].
Following the recommendations of the Cochrane Handbook of Systematic Reviews for interventions, we assessed the risk of bias for RCTs using the Cochrane risk-of-bias tool for randomised trials (RoB2) and the ROBINS-I tool was used to assess for bias in qRCTs [15,16].
Results
Study Selection
Utilizing the 4 databases and the search strategy described above, 112 studies were initially identified. No additional records were identified through other sources. 39 studies were found to be duplicates and instantly excluded. Further screening of titles and abstracts excluded 63 studies of the remaining 73, due to not meeting the eligibility criteria of this study. The full texts of the 10 studies remaining were screened once again, 5 from PubMed and 5 from EMBASE. This review included 8 eligible studies that were identified from the remaining 10. These 2 studies were excluded due to non-specific description of patient fracture types and the other didn’t describe a FES criterion for diagnosis. The PRISMA flow diagram below (Figure 1) provides an overview of the search strategy and process of selected studies.
Utilizing the 4 databases and the search strategy described above, 112 studies were initially identified. No additional records were identified through other sources. 39 studies were found to be duplicates and instantly excluded. Further screening of titles and abstracts excluded 63 studies of the remaining 73, due to not meeting the eligibility criteria of this study. The full texts of the 10 studies remaining were screened once again, 5 from PubMed and 5 from EMBASE. This review included 8 eligible studies that were identified from the remaining 10. These 2 studies were excluded due to non-specific description of patient fracture types and the other didn’t describe a FES criterion for diagnosis. The PRISMA flow diagram below (Figure 1) provides an overview of the search strategy and process of selected studies.
Study Characteristics
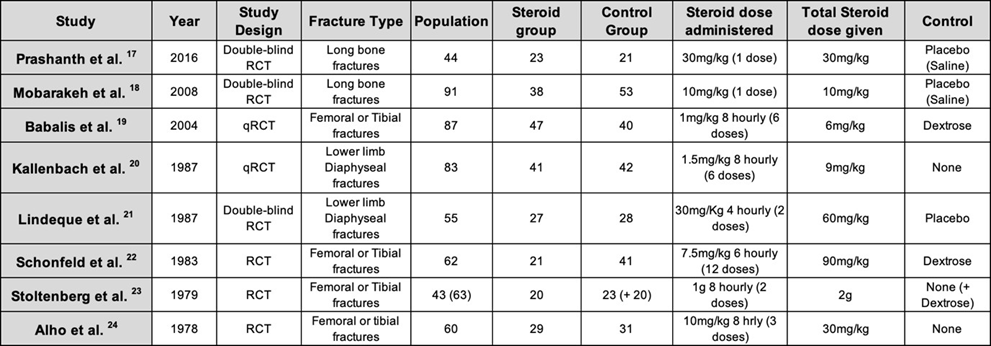
This review included 8 studies [17, 24]; 6 RCTs and 2 qRCTs, that were published between 1978 and 2016. The populations of the studies ranged from 43 to 91 with the majority dividing them equally into two groups, the steroid group and the control group, with the study by Schonfeld et al [22] being the exception, having double the population size in the control group. Studies used similar inclusion and exclusion criteria; however, there was still a significant difference in variation in the characteristics of the eight studies included, summarized in Table 2. Although the same corticosteroid were used for all studies, IV Methylprednisolone Sodium Succinate, the dose per kg and duration, differed significantly. This resulted in a wide range of total steroid administered, from as low as 6mg/kg to as high as 90mg/kg, with one study giving 2g regardless of the weight [23]. The controls used also differed significantly. Two of the eight studies had patients randomised by alternate sequence and hence regarded as quasi randomised controlled trials (qRCT). The majority of the patients selected in the studies had comparable fracture patterns with a young male cohort. Significant variation was also present in the diagnostic criteria for FES, while similar arterial oxygen partial pressures used for the cut off value for hypoxia (Table 3).
This review included 8 studies [17, 24]; 6 RCTs and 2 qRCTs, that were published between 1978 and 2016. The populations of the studies ranged from 43 to 91 with the majority dividing them equally into two groups, the steroid group and the control group, with the study by Schonfeld et al [22] being the exception, having double the population size in the control group. Studies used similar inclusion and exclusion criteria; however, there was still a significant difference in variation in the characteristics of the eight studies included, summarized in Table 2. Although the same corticosteroid were used for all studies, IV Methylprednisolone Sodium Succinate, the dose per kg and duration, differed significantly. This resulted in a wide range of total steroid administered, from as low as 6mg/kg to as high as 90mg/kg, with one study giving 2g regardless of the weight [23]. The controls used also differed significantly. Two of the eight studies had patients randomised by alternate sequence and hence regarded as quasi randomised controlled trials (qRCT). The majority of the patients selected in the studies had comparable fracture patterns with a young male cohort. Significant variation was also present in the diagnostic criteria for FES, while similar arterial oxygen partial pressures used for the cut off value for hypoxia (Table 3).
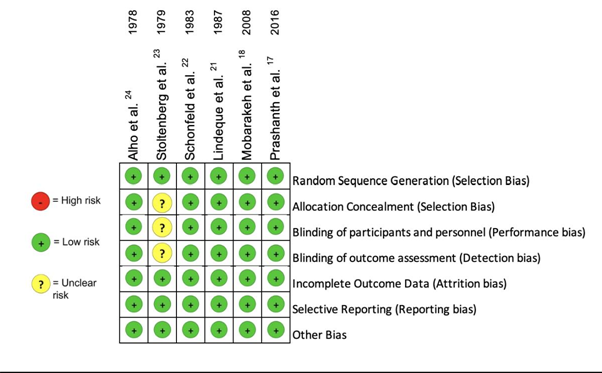
Quality Assessment and Risk of Bias
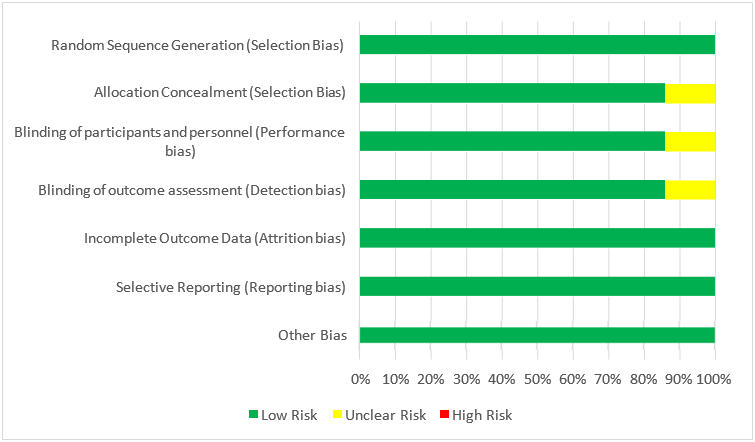
Each of the eight studies [17, 24] included had comprehensive designs with minimal risk of bias [15, 16]. The risk of bias for RCTs (Figure 2) and qRCTs (Table 4) were assessed according to the RoB2 and the ROBINS-I tools accordingly. Along with the Cochrane Risk of Bias Tool, a Risk of Bias Graph was produced and included in this systematic review to reveal the risk of bias not only in individual studies, but also across all studies as a percentage.
Each of the eight studies [17, 24] included had comprehensive designs with minimal risk of bias [15, 16]. The risk of bias for RCTs (Figure 2) and qRCTs (Table 4) were assessed according to the RoB2 and the ROBINS-I tools accordingly. Along with the Cochrane Risk of Bias Tool, a Risk of Bias Graph was produced and included in this systematic review to reveal the risk of bias not only in individual studies, but also across all studies as a percentage.
All RCTs described a well-defined primary outcome that was the same for all RCTS included in this study; however, secondary outcomes differed slightly besides hypoxaemia which was investigated throughout. Despite many studies having different criteria for diagnosing FES, the respective criteria were specifically described and argued for, with each study detailing their respective PICO components. Population sizes were small, ranging from 43 to 91, with the majority having equal steroid vs control groups. With regard to the six RCTs, randomisation was clearly stated in these studies, with alternate sequence randomization utilized in the qRCTs. Random sequence generation was constructed using sealed envelopes18, while the remaining RCTs did not specify. Double blinding were described in four studies [17, 18, 21, 22,] though allocation concealment reporting was at times vaguely reported as demonstrated below in Figure 2. Studies reported follow up periods ranging from 48 hours to 5 days, which was sufficient for detection and treatment monitoring of FES; however, it limited analysis of secondary outcomes that may take longer to be diagnosed, thus at risk of reporting bias. The study by Stoltenberg et al [23] lacked clarity and specificity regarding the blinding of personnel, hence potentially leading to bias. All studies provided thorough reporting effects of the intervention.
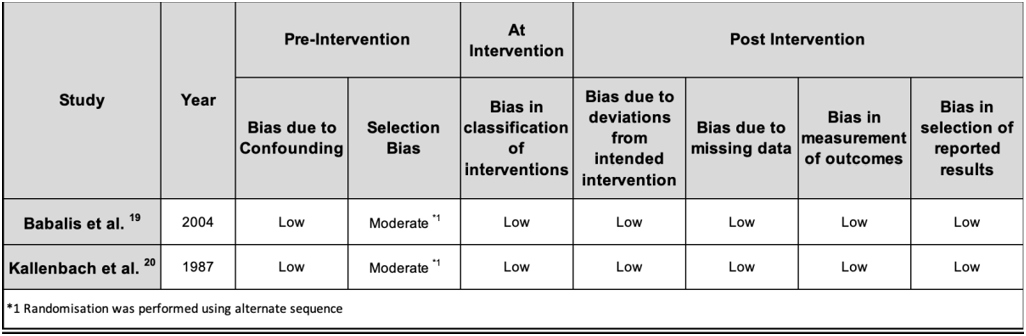
The two qRCTs [19, 20] also followed a focused question, with similar secondary outcomes, specifying their PICO components. They were deemed as qRCTs as the patients were assigned into groups using alternate sequencing. The study by Babalis et al was blinded; however, the study by Kallenbach et. al was not and thus may have contributed to some selection bias. Bias due to confounding and post-intervention bias was minimal in the two studies. Overall, the two RCTs were of good quality as can be seen in the Table 4 below.
Results of Individual Studies
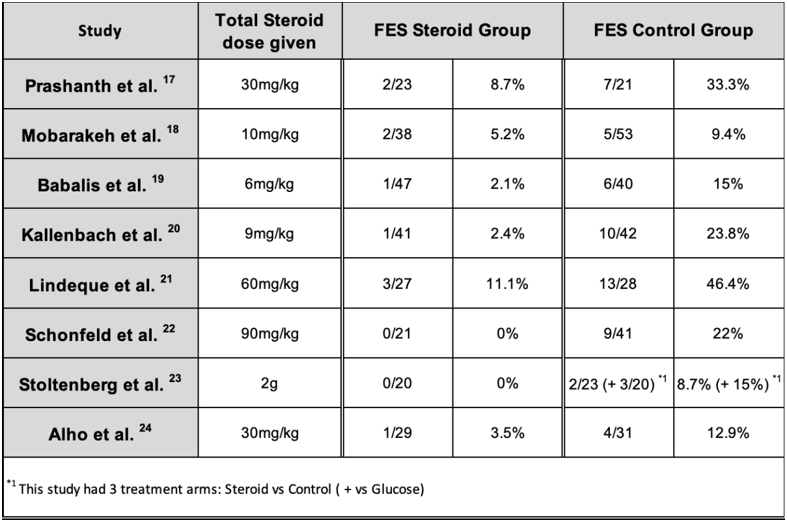
Alho et al. The authors described the patients being diagnosed with either a fulminant variety of FES or a less severe form of FES, with only the former taken into consideration in the tables above and for the calculations below. 1 steroid treated patient and 4 control patients developed fulminant FES, whilst 1 steroid treated patient and 11 control patients developed the less severe variety. The authors calculated a fisher exact probability of 0.0003. [24]
Stoltenberg et al. This study utilized hypoxia, neurological signs, and irritability to diagnose FES, and despite having the broadest criteria from all other studies, only 2 patients in the control group and none in the steroid group developed FES. The authors had a third group, a control group given dextrose where 3 developed FES. This was found to be statistically insignificant; however, it is important to take into consideration that the population size was small. Despite these findings, it was found that 15 patients developed hypoxia in the control groups compared to 3 in the steroid group, this was deemed to be statistically significant. [23]
Schonfeld et al. Using the specific scoring system outlined above, this study found that 0 out of 21 corticosteroid treated patients developed FES compared to the 9 out of 41 in the control group that was given dextrose. Using Fisher’s exact test, this was found to be statistically significant (P < 0.05). Contrary to the findings of the Stoltenberg et al., the relationship of hypoxia in both groups was not statistically significant. [22]
Lindeque et al. This was the first study where the authors created their own diagnostic criteria which lead to its widespread use in the medical field and subsequent studies. FES was diagnosed in 3 patients from the steroid receiving group and 13 in the control population. It was also found that steroids had a substantial role in decreasing the incidence of hypoxia, 6 in the trial and 16 in the control. These findings were both statistically significant, by which the authors used the McNemar test (p < 0.01) for FES and Chi squared test (X2 < 0.025) for hypoxaemia. [21]
Kallenbach et al.: There was a significant difference (p < 0.01) in FES diagnosed in this study when comparing the steroid group (1/41) and the control group (10/42). The effect of prophylactic methylprednisolone also had a significant effect on both isolated severe arterial hypoxaemia and the overall incidence of the arterial hypoxaemia, p < 0.01 and p < 0.05 respectively. [20]
Babalis et al. Similar to the study by Schonfeld et al., the authors developed a scoring system for FES outlined above. Despite having this scoring system and one of the largest populations from the 8 studies, only 1 out of 47 steroid treated patients and 6 out of 40 control patients developed FES. Although the difference and conclusion that steroids did indeed have an effect, it was found to be statistically insignificant using the Pearson’s x2 test, producing a value of 0.079. It was postulated by the authors that if the population size was larger, the difference would have been statistically significant. Contrary to these findings, when evaluating hypoxaemia on the 2nd and 3rd days from admission, the difference was significant as none of the patients in the steroid group had a PO2 < 60mmHg as opposed to the 13 patients in the control group (p-value of 0.008). [19]
Mobarakeh et al. The authors utilized Gurd’s criteria for diagnosing FES and found that there were 2 cases of FES in the trial group and 5 cases in the control group from a total population of 91. With regards to isolated hypoxaemia, 1 patient from the steroid group compared to 8 in the control group. This study also looked at mean arterial oxygen pressures. From this data and applying different statistical calculations, there was no statistically significant difference between the steroid and control groups when comparing FES cases (X2 0.461), hypoxic patients (P 0.07, Fisher’s exact test) and mean arterial pressures (P 0.386, Mann-Whitney U test). [18]
Prashanth et al. In this study, Lindeque’s criteria was used for diagnosing FES. 2 out of 23 from steroid receiving group and 7 out of 21 from the control group were diagnosed with FES. This was found to be statistically significant with a p-value of 0.043. The authors did not specify whether other patients besides the ones diagnosed with FES developed hypoxaemia, however they did find that ventilatory support was necessary for a longer duration in the control group (9.25 days) compared to the steroid group (7.33 days). [17]
Primary Outcome
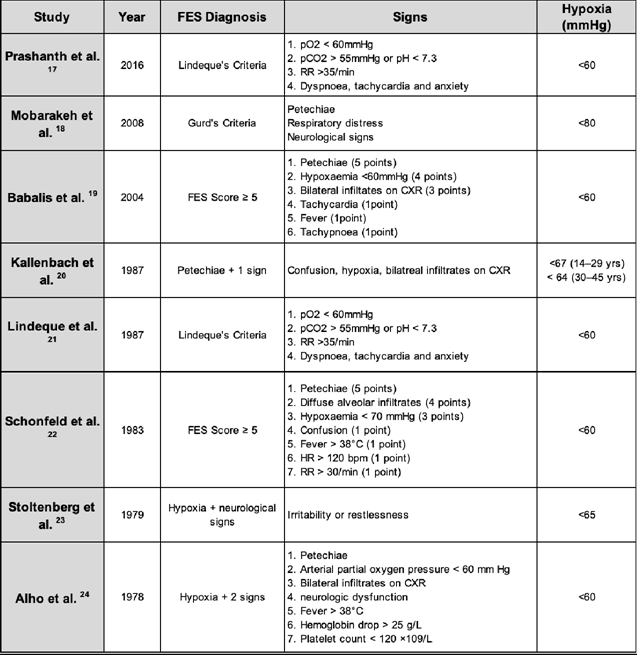
The eight studies analysed a total population of 545 patients, where 246 had been prescribed steroids and 299 formed the control groups. Although the dose of corticosteroid and frequency varied considerably, all studies administered Methylprednisolone Sodium Succinate intravenously. Most clinicians use Gurd’s criteria for diagnosing FES in clinical day to day practice; however, only the study by Mobarakeh et al. utilized these criteria. Five [18, 20, 22, 24] out of 8 studies included the cutaneous manifestation of petechiae as part of diagnosing FES. Majority of the studies had detailed specific diagnostic signs for FES, with two studies [19, 22] allocating points to each sign and developing a scoring system for diagnosis. These are outlined in Table 3.
The eight studies analysed a total population of 545 patients, where 246 had been prescribed steroids and 299 formed the control groups. Although the dose of corticosteroid and frequency varied considerably, all studies administered Methylprednisolone Sodium Succinate intravenously. Most clinicians use Gurd’s criteria for diagnosing FES in clinical day to day practice; however, only the study by Mobarakeh et al. utilized these criteria. Five [18, 20, 22, 24] out of 8 studies included the cutaneous manifestation of petechiae as part of diagnosing FES. Majority of the studies had detailed specific diagnostic signs for FES, with two studies [19, 22] allocating points to each sign and developing a scoring system for diagnosis. These are outlined in Table 3.
Using these diagnostic criteria, only 10 out of 246 steroid-treated patients developed FES, compared to the 59 out of 299 control patients (Table 5). Using the unpaired t-test, this resulted in a p-value <0.05. Using this pooled data, the absolute risk reduction (ARR) was 0.156, resulting in a numbers needed to treat (NNT) value of 6.41. Therefore, only 6 patients needed to be treated with corticosteroids to prevent 1 case of FES.
Secondary Outcomes
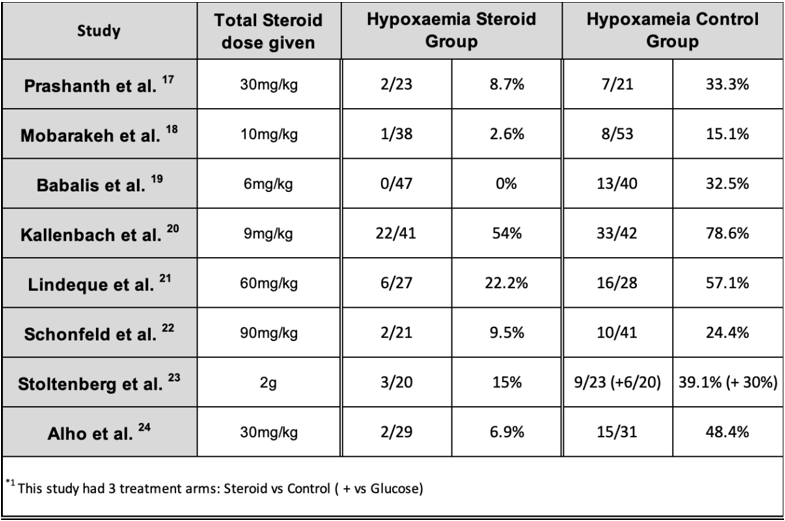
Authors from 7 out of 8 studies also assessed the effect of corticosteroids in preventing the incidence of hypoxaemia, with the exception being Prashanth et al. The latter did not distinguish if there were any patients that developed hypoxia alone and only described the hypoxic patients that fell under the patients diagnosed with FES. Therefore, the number of hypoxic patients found in that study is listed (Table 6) as the same number of patients with FES; however, this may have an element of reporting bias. Different studies had different cut off points for hypoxia that ranged from
Authors from 7 out of 8 studies also assessed the effect of corticosteroids in preventing the incidence of hypoxaemia, with the exception being Prashanth et al. The latter did not distinguish if there were any patients that developed hypoxia alone and only described the hypoxic patients that fell under the patients diagnosed with FES. Therefore, the number of hypoxic patients found in that study is listed (Table 6) as the same number of patients with FES; however, this may have an element of reporting bias. Different studies had different cut off points for hypoxia that ranged from
The use of prophylactic corticosteroids had no effect on mortality rates, nor did it influence infections rates. None of the studies reported avascular necrosis; however, patient follow up was short, with a maximum of 5 days leading to a high risk of reporting bias since avascular necrosis can take months to develop. [25]
Discussion
FES is a life-threatening condition, affecting a significant number of patients with single or multiple long bone fractures. The use of prophylactic corticosteroids to prevent FES is a topic of debate in current clinical practice. The study of corticosteroids in treating fat embolism dates back to the 1960s through animal studies [26]. Further animal studies revealed the benefit of steroids in preventing FES in trauma scenarios and improves oxygenation by limiting lung damage from the fat globules [27,28]. This influenced further studies to determine the effect of prophylactic corticosteroids in preventing FES in patients with long bone fractures.
Understanding the pathophysiology behind FES aids in appreciating why corticosteroids have been found to be effective. Fat globules enter the peripheral circulation after sustaining a fracture, which then reach the respiratory and cerebral blood supply. Within the capillaries, they then cause blockage followed by a chemical phase. Free fatty acids induce local inflammation, leading to endothelial damage by neutrophils [29,30]. Although the exact mechanism of Methylprednisolone is still under debate, studies suggested it has a role in inhibiting the complement-mediated aggregation of neutrophils in the pulmonary circulation, hence preventing endothelial damage [20].
A recent nonrandomized prospective control trial evaluated the efficacy and safety of inhalational Ciclesonide (CIC) in FES prevention and hypoxaemia treatment in trauma patients with isolated skeletal injuries. Inhalational steroids have less systemic effects and are already commonly utilized for respiratory conditions, such as asthma and ARDS. This study had similar inclusion criteria to the 8 studies in this review, which resulted in a population of 70 patients, 35 in a steroid group and 35 in a control group. The author’s used Gurd’s criteria to diagnose FES and used a cut off value of
All 8 studies investigated intravenous Methylprednisolone Sodium Succinate; however, dosing regimens differed in dose per kg and duration. Corticosteroids were shown to have a beneficial effect in preventing both FES and hypoxaemia in patients with long bone fractures, irrespective of whether high- or low-dose treatments were used. However, the low-dose groups resulted in better outcomes, which correlates with findings of ARDS RCTs [32].
Limitations
Only 8 studies that fulfilled the requirements stated earlier were identified, out of which 6 were RCTs, dating back to the 1970s. The sample sizes of these studies were small, with the largest study involving 91 participants [18]. While this review did not find a substantial level of bias in the studies we examined, some bias was still observed. The collected data displayed a significant amount of variation, therefore a metanalysis could not be carried out.
Only 8 studies that fulfilled the requirements stated earlier were identified, out of which 6 were RCTs, dating back to the 1970s. The sample sizes of these studies were small, with the largest study involving 91 participants [18]. While this review did not find a substantial level of bias in the studies we examined, some bias was still observed. The collected data displayed a significant amount of variation, therefore a metanalysis could not be carried out.
Conclusion
There is evidence indicating that the use of corticosteroids may have a positive effect in preventing FES and hypoxia in patients with long bone fractures. Furthermore, it does not seem to have a significant impact on mortality rates nor does it appear to increase the risk of infection. Nonetheless, it is important to acknowledge that the trials conducted so far have certain limitations in their methodologies, mainly sample size and follow up period length. Therefore, a large-scale randomized trial is needed to provide further confirmation and validation of these findings.
References
- Chan KM, Tham KT, Chiu HS, et al. (1984). Posttraumatic fat embolism: its clinical and subclinical presentations. J Trauma. 24: 45-9.
- Riseborough EJ, Herndon JH. (1976). Alterations in pulmonary function, coagulation and fat metabolism in patients with fractures of the lower limbs. Clin Orthop Relat Res 115: 248-67.
- Fabian TC, Hoots AV, Stanford DS, et al. (1990). Fat embolism syndrome: prospective evaluation in 92 fracture patients. Crit Care Med18: 42-6.
- Bulger EM, Smith DG, Maier RV, et al. (1997). Fat embolism syndrome. A 10-year review. Arch Surg 132: 435-9.
- Wenda K,Henrichs KJ,Biegler M,etal. (1989). Recordingofbone-marrow embolism during intramedullary nailing of the femur by trans- oesophageal echocardioy. Unfallchirurgie 15: 73-6.
- Gurd AR, Wilson RI. (1974). The fat embolism syndrome. J Bone Joint Surg Br 56: 408-16.
- Schonfeld SA, Ploysongsang Y, DiLisio R, et al. (1983). Fat embolism pro- phylaxis with corticosteroids. A prospective study in high-risk patients. Ann Intern Med 99: 438-43.
- Behrman SW, Fabian TC, Kudsk KA, et al. [1990]. Improved outcome with femur fractures: early vs. delayed fixation. J Trauma 30: 792-7.
- Pinney SJ,KeatingJ F,Meek RN. (1998). Fat embolism syndrome in isolated femoral fractures: Does timing of nailing influence incidence? Injury 29: 131-3.
- Sage R, Tudor R. [1958]. Treatment of fat embolism with heparin. BMJ i: 1160-1.
- Gossling HR, Pellegrini VD Jr. [1982]. Fat embolism syndrome: a review of the pathophysiology and physiological basis of treatment. Clin Orthop Relat Res 68-82.
- Meduri GU, Golden E, Freire AX, et al. [2007]. Methylprednisolone infu- sion in early severe ARDS. Results of a randomized controlled trial. Chest 131:954-63.
- PRISMA-P Group, Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, et al. [2015]. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systematic Reviews. 4(1): 1.
- Critical Appraisal Skills Programme. CASP Checklists [Internet]. [2019]. Available from: https://casp-uk.net/casp-tools-checklists/
- Sterne JAC, Savovic J, Page M, Elbers R, Blencowe N, Boutron I, et al. [2019], RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 366(I4898).
- Sterne JAC, Hernan M, Reeves B, Savovic J, Berkman N, Viswanathan M, et al. [2016]. ROBINS-I: a tool for assessing risk of bias in non-randomized studies of interventions. BMJ. 355(I4919).
- Prashanth N, Neeta PN, Amit RU, Shilpa K. [2016]. Effect of intravenous methylprednisolone in prevention of arterial hypoxemia due to fat embolism syndrome in patients with long bone fractures of lower limb - A double blind randomized trial. Anaesth Pain & Intensive Care 20(3): 290-294.
- Mobarakeh MK, Saied AR, Scott RK (2008). Efficacy of Corti- costeroid in Prevention of fat embolism syndrome in patients with long bone fracture. Ir J Med Sc 33(4).
- Babalis GA, Yiannakopoulos CK, Karliaftis K, Antonogiannakis E (2004). Prevention of posttraumatic hypoxaemia in isolated lower limb long bone fractures with a minimal prophylactic dose of corticosteroids. Injury 35(3): 309–317
- Kallenbach J, Lewis M, Zaltzman M, Feldman C, Orford A, Zwi S. [1987]. ‘Low-dose’ corticosteroid prophylaxis against fat embolism. J Trauma. 27:1173–6.
- Lindeque BG, Schoeman HS, Dommisse GF, Boeyens MC, Vlok AL. [1987]. Fat embolism and the fat embolism syndrome: a double-blind therapeutic study. J Bone Joint Surg Br. 69: 128–31.
- Schonfeld SA, Ploysongsang Y, DiLisio R, et al. [1983]. Fat embolism pro- phylaxis with corticosteroids. A prospective study in high-risk patients. Ann Intern Med 99:438-43.
- Stoltenberg JJ, Gustilo RB. [1979]. The use of methylprednisolone and hypertonic glucose in the prophylaxis of fat embolism syndrome. Clin Orthop Relat Res. (143): 211-21.
- Alho A, Saikku K, Eerola P, Koskinen M, Hamalainen M (1978). Corticosteroids in patients with a high risk of fat embolism syndrome. Surg Gyn Obst 147(3): 358–362.
- McKee MD, Waddell JP, Kudo PA, et al. [2001]. Osteonecrosis of the femoral head in men following short-course corticosteroid therapy: a report of 15 cases. Can Med Assoc J 164: 205-6.
- Ashbaugh DG, Petty TL (1966). The use of corticosteroids in the treatment of respiratory failure associated with massive fat embolism. Surg Gynecol Obstet 123: 493–500.
- Rokkanen P, Alho A, Avikainen V., et al. (1974). The efficacy of corticosteroids in severe trauma. Surg Gynecol Obstet 138: 69–73
- Kreis WR, Lindenauer S, Dent TL. (1973). Corticosteroids in experimental fat embolization. J Surg Res 14: 238
- Fabian TC. (1993). Unraveling the fat embolism syndrome. N Engl J Med 329: 961–963
- Gossling HR, Pellegrini VD Jr. (1982). Fat embolism syndrome. Clin Orthop 165: 68–82
- Sen RK, Prakash S, Tripathy SK, Agarwal A, Sen IM. [2017]. Inhalational Ciclesonide found beneficial in prevention of fat embolism syndrome and improvement of hypoxia in isolated skeletal trauma victims. Eur J Trauma Emerg Surg. Jun;43(3): 313-318.
- Meduri GU, Golden E, Freire AX, et al. [2007]. Methylprednisolone infu- sion in early severe ARDS. Results of a randomized controlled trial. Chest; 131: 954-63.
Citation: Mr Luca Calleja, Dr Preetisha Chadee and Dr Mumraiz Naqshband. (2025). A Systematic Review of Prophylactic Corticosteroids in the Prevention of Fat Embolism Syndrome Following Long Bone Fractures. Journal of Orthopaedic and Trauma Care 6(1).
Copyright: © 2025 Luca Calleja. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.