Review Article
Volume 6 Issue 1 - 2025
The use of preoperative Fascia Iliaca Compartment Block (FICB) as an effective form of analgesia in adult patients with hip fractures: A systematic review
1MD, MRCS, MSc. Department of Orthopaedics, Trauma and Sports Medicine, Mater Dei Hospital, Msida Malta
2MSc, BSc (Hons), RN. Department of Orthopaedics, Trauma and Sports Medicine, Mater Dei Hospital, Msida Malta
2MSc, BSc (Hons), RN. Department of Orthopaedics, Trauma and Sports Medicine, Mater Dei Hospital, Msida Malta
*Corresponding Author: Isaac Balzan, MD, MRCS, MSc. Department of Orthopaedics, Trauma and Sports Medicine, Mater Dei Hospital, Msida Malta.
Received: January 15, 2025; Published: January 31, 2025
Abstract
Background: Hip fractures in the elderly are linked to high morbidity and mortality. Pain control is crucial for improving outcomes, and traditional treatments include paracetamol and opiates. Fascia Iliaca Compartment Block (FICB) is a promising alternative that provides effective analgesia with minimal complications.
Aims: This review aimed to evaluate the efficacy and safety of FICB, as well as its potential to reduce opioid use and the incidence of delirium in hip fracture patients.
Methods: A comprehensive search of Cochrane, Medline, PubMed, and Embase identified relevant studies on FICB in hip fracture patients.
Results: Ten studies, including six randomized controlled trials, three non-randomized studies, and one observational study, were analyzed with 1,520 patients. Seven studies reported significant pain relief after FICB. Six of eight studies found a reduction in opioid consumption, with no complications noted. Results on delirium reduction were inconsistent.
Conclusion: FICB is a safe and effective pain management option for hip fracture patients, offering comparable or superior analgesia to opioids and NSAIDs. It reduces the need for additional analgesics, thus minimizing side effects. The effect of FICB on delirium remains unclear and warrants further research.
Keywords: Fascia-iliaca compartment block; FICB; Hip Fracture; Analgesia
Abbreviations: FICB: Fascia-iliaca Compartment Block; LA: Local anaesthetic; RCT: Randomized control study; ED: Emergency department; IM: Intramuscular; IV: Intravenous; LOS: Length of stay; NSAID: Non-steroidal anti-inflammatory drug; NRS: Numerical rating score; PO: Per Oral; SPA: Standard Preoparative analgesia; US: Ultrasound; VAS: Visual Analogue Score
Introduction
The worldwide population age is steadily increasing and, with it, an increase in the number of hip fractures. Hip fractures are considered a global epidemic in several countries [1]. Research by Cooper et al. [2] projected that the number of global hip fractures would increase from 1.66 million in 1990 to 6.26 million by 2050. The increase in hip fractures with age can be accredited to a decrease in bone mineral mass and an increased risk of falls in the elderly. The incidence of hip fractures is around twice as common in women as in men, with mechanical falls being the most common cause [3]. Hip fractures are associated with high mortality and morbidity rates, including disability and institutionalization [4].
Adequate pain control is an essential component of hip fracture management. Suboptimal pain control is associated with worse outcomes and increased complications [5]. Historically, patients were given analgesia in the form of a combination of paracetamol, weak opioids and morphine. More recently, regional nerve blocks have emerged as an effective adjunct to traditional methods [5]. The National Institute for Health and Care Excellence (NICE) also recommends the use of nerve blocks to improve patient outcomes. (6) Fascia Iliaca compartment block (FICB) is a more commonly used regional block technique and was first described by Dalens et al. (7) in 1989. FICB involves administering a large volume of dilute local anaesthetic (LA) into the fascia iliaca compartment, thereby theoretically anaesthetising the femoral, obturator, lateral cutaneous and genitofemoral nerves as well as other elements of the lumbar plexus, as the anaesthetic tracks caudally. Dalens et al. (7) demonstrated that FICB produced a more effective sensory blockade with no complications reported.
FICB has several advantages over other regional blocks and systemic analgesia; the procedure can be performed with ease using the “two-pop” technique or under ultrasound guidance and is safe with no absolute contraindications to be noted. Clotting disorders, allergy to LA, local infection and an un-cooperative patient are relative contraindications [7,8]. Despite the apparent benefits of FICB, its application is still not prevalent.
The scope of this review is to provide an overview of the literature and compare the use of FICB in hip fracture patients with that of conventional analgesia by answering the question “In adult patients (>18 years) with femoral neck fractures, does the administration of a preoperative FICB provide an effective and safe means of analgesia when compared to conventional analgesia?”
The aims of this review were to assess the analgesic efficacy of a preoperative FICB in adult patients with hip fractures, to determine whether FICB is a safe mode of analgesia and to investigate whether FICB can decrease the consumption of conventional analgesia such as opiates and the complications associated with them.
Materials and Methods or Experimental Procedures
Review Registration
This systematic review was registered on the international prospective register of systematic reviews, PROSPERO with registration number CRD42021232877.
This systematic review was registered on the international prospective register of systematic reviews, PROSPERO with registration number CRD42021232877.
Data Sources and Search Strategy
Between December 2020 and February 2021, a comprehensive search for eligible studies was conducted across electronic databases, including COCHRANE, PubMed, Embase, and Medline. The search strategy, based on PICO components, utilized free-text terms, keywords, MeSH terms, and Boolean operators to identify relevant studies. References of selected studies were also screened for eligibility. To minimize bias, the search strategy and results were reviewed and validated by a second reviewer.
Between December 2020 and February 2021, a comprehensive search for eligible studies was conducted across electronic databases, including COCHRANE, PubMed, Embase, and Medline. The search strategy, based on PICO components, utilized free-text terms, keywords, MeSH terms, and Boolean operators to identify relevant studies. References of selected studies were also screened for eligibility. To minimize bias, the search strategy and results were reviewed and validated by a second reviewer.
Inclusion and Exclusion Criteria
Study selection followed specific inclusion and exclusion criteria, focusing on randomized controlled trials (RCTs), quasi-RCTs (q-RCTs), and observational studies comparing the analgesic efficacy of preoperatively administered FICB to traditional analgesia in adults with isolated hip fractures. Various study designs were included due to an overall lack of available research. Eligible studies were written in English with accessible full texts, and no date restrictions were applied due to the expected scarcity of relevant literature. Traditional analgesia included commonly used methods such as paracetamol, NSAIDs, mild opiates, and morphine, excluding other nerve or regional blocks.
Study selection followed specific inclusion and exclusion criteria, focusing on randomized controlled trials (RCTs), quasi-RCTs (q-RCTs), and observational studies comparing the analgesic efficacy of preoperatively administered FICB to traditional analgesia in adults with isolated hip fractures. Various study designs were included due to an overall lack of available research. Eligible studies were written in English with accessible full texts, and no date restrictions were applied due to the expected scarcity of relevant literature. Traditional analgesia included commonly used methods such as paracetamol, NSAIDs, mild opiates, and morphine, excluding other nerve or regional blocks.
Primary and Secondary Outcomes
The primary outcome for this review was pain relief, as measured by a pain measurement tool, after administration of FICB in adult patients with an isolated hip fracture. Secondary outcomes for this review included the incidence of any adverse effects from FICB administration, additional analgesia consumption, particularly opioids, and incidence of delirium.
The primary outcome for this review was pain relief, as measured by a pain measurement tool, after administration of FICB in adult patients with an isolated hip fracture. Secondary outcomes for this review included the incidence of any adverse effects from FICB administration, additional analgesia consumption, particularly opioids, and incidence of delirium.
Data Collection, Screening and Management
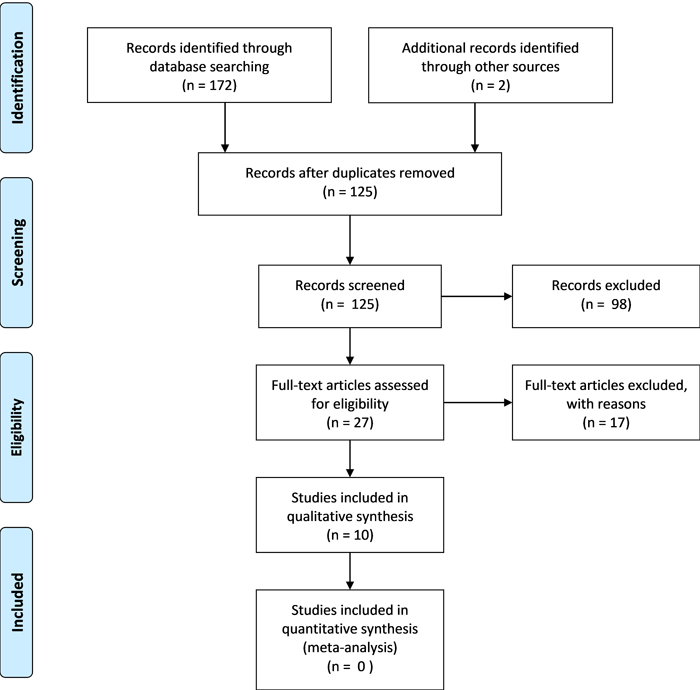
A thorough search for eligible studies was conducted following the established search strategy and inclusion/exclusion criteria. The primary reviewer initially performed the search independently, followed by collaboration with a second reviewer. There were no discrepancies so a third reviewer was not necessary. Titles and abstracts were screened for eligibility, and relevant study details, including PICO components, were documented in a custom Microsoft Excel sheet for data management. Excluded studies and reasons for exclusion were recorded, and duplicates were marked. For unclear cases, full texts were accessed and reviewed. A librarian assisted in obtaining inaccessible full texts. The process is summarized in a PRISMA(9) flow diagram (figure 1).
A thorough search for eligible studies was conducted following the established search strategy and inclusion/exclusion criteria. The primary reviewer initially performed the search independently, followed by collaboration with a second reviewer. There were no discrepancies so a third reviewer was not necessary. Titles and abstracts were screened for eligibility, and relevant study details, including PICO components, were documented in a custom Microsoft Excel sheet for data management. Excluded studies and reasons for exclusion were recorded, and duplicates were marked. For unclear cases, full texts were accessed and reviewed. A librarian assisted in obtaining inaccessible full texts. The process is summarized in a PRISMA(9) flow diagram (figure 1).
Data Analysis and Quality Assessment
The data extracted from the included studies was evidently highly heterogeneous. Thus, this data did not lend itself to meta-analysis and a narrative analysis was performed. Quality appraisal of the included studies was guided by the Critical Appraisal Skills Programme (CASP) Tool(10) for both the RCTs and Non-RCTs.
The data extracted from the included studies was evidently highly heterogeneous. Thus, this data did not lend itself to meta-analysis and a narrative analysis was performed. Quality appraisal of the included studies was guided by the Critical Appraisal Skills Programme (CASP) Tool(10) for both the RCTs and Non-RCTs.
Risk of Bias
The Cochrane risk-of-bias tool for randomised trials (RoB2)[11] was used to assess risk of bias for RCTs while the ROBINS-I [12] tool was used to assess for bias in Non-randomised RCTs, as recommended by the Cochrane Handbook of Systematic Reviews for interventions.
The Cochrane risk-of-bias tool for randomised trials (RoB2)[11] was used to assess risk of bias for RCTs while the ROBINS-I [12] tool was used to assess for bias in Non-randomised RCTs, as recommended by the Cochrane Handbook of Systematic Reviews for interventions.
Results
Study Selection
The search identified 174 potentially eligible studies across four databases. After removing 49 duplicates, 125 studies remained. Screening of titles and abstracts excluded 98 ineligible studies. The full texts of the remaining 27 studies (11 from Cochrane, 7 each from Embase and PubMed, and 2 from references) were reviewed, resulting in 10 eligible studies included in the review. Reasons for exclusion included inaccessible full texts (n=6), inappropriate primary outcomes (n=6), absence of a control group (n=3), and incorrect study design (n=2). The process is summarized in figure 1.
The search identified 174 potentially eligible studies across four databases. After removing 49 duplicates, 125 studies remained. Screening of titles and abstracts excluded 98 ineligible studies. The full texts of the remaining 27 studies (11 from Cochrane, 7 each from Embase and PubMed, and 2 from references) were reviewed, resulting in 10 eligible studies included in the review. Reasons for exclusion included inaccessible full texts (n=6), inappropriate primary outcomes (n=6), absence of a control group (n=3), and incorrect study design (n=2). The process is summarized in figure 1.
Study Characteristics
This review included ten studies published between 2007 and 2020: six RCTs, three non-RCTs, and one observational study. Study populations ranged from 30 to 725 participants, with most dividing intervention and control groups equally, except for one study by Garlich et al. [13], which used a larger historical control group. All studies compared FICB to other analgesia forms, with variations in the type and dose of local anesthetics (bupivacaine, levobupivacaine, and ropivacaine in three studies each, and mepivacaine in one study). Seven studies [14–20] administered a single-dose FICB, tw [21,22] used continuous infusion via a catheter, and one [13] utilized both methods based on the physician's discretion.
This review included ten studies published between 2007 and 2020: six RCTs, three non-RCTs, and one observational study. Study populations ranged from 30 to 725 participants, with most dividing intervention and control groups equally, except for one study by Garlich et al. [13], which used a larger historical control group. All studies compared FICB to other analgesia forms, with variations in the type and dose of local anesthetics (bupivacaine, levobupivacaine, and ropivacaine in three studies each, and mepivacaine in one study). Seven studies [14–20] administered a single-dose FICB, tw [21,22] used continuous infusion via a catheter, and one [13] utilized both methods based on the physician's discretion.
Blocks were administered by trained physicians, except in one [14] study where it was unspecified. Single-shot FICB used a landmark technique, while catheter-based methods employed ultrasound guidance. Pain relief was the primary outcome in all studies, measured using respective pain scores. Secondary outcomes varied, and accessory analgesia was reported in all but one [14] study. Study characteristics are detailed in Table 1.
| Author and year | Study Design | Population (FICB:NO FICB) |
Age | Intervention | Comparison | Other common analgesia | Setting | Performed by | Technique | Primary outcome - Pain relief | Secondary outcomes |
| Foss et al.(15) 2007 |
Double-Blind RCT | 48 (24:24) |
69 - 88 | FICB with 40ml 1% Mepivacaine + Epinephrine 1:200,000 + Placebo IM injection of isotonic saline |
Placebo FICB with 0.9% saline + IM injection of 0.1mg/kg, 5mg/ml morphine |
1. 1g Paracetamol PO 2. 2.5mg IV morphine PRN for persistent pain |
ED | Junior anaesthetists with basic instructions on how to perform block | Landmark technique | Pain score at rest and on movement at 30, 60 & 180 mins on 0-10 VAS Pain relief at 30 mins after block on 0-10 VAS Pain on repositioning at least 60 mins after block on 0-10 VAS |
Additional morphine consumption |
| Monzon et al.(14) 2010 | Double-Blind RCT | 154 (92:62) |
No Range Given | FICB with 0.3ml/kg 0.25% Bupivacaine + Placebo 5% dextrose iv |
Placebo FICB with 0.9% saline + IV injection of NSAID |
/ | ED | Not specified | Landmark technique | Pain score (0-10 VAS) at 15 min, 2 hrs & 8hrs | 1. Parameters 2. Any complications |
| Hanna et al.(16) 2014 | Non-RCT | 104 (52:52) |
25 - 100 | FICB with 0.25% Levobupivacaine: 20ml (<40kg), 30ml (40-80kg) or 40ml (>80kg) |
Traditional analgesia (WHO pain ladder) | / | ED | Trained orthopaedic trainees or trained ED physicians | Landmark technique | Pain score (0-10 VAS) on movement at baseline, 15mins, 2,8,16 and 24 hrs | 1. Time to initial analgesia 2. Total preoperative dose of analgesia 3. Any complications |
| Williams et al.(17) 2016 | Non-RCT | 119 (50:69) |
58 - 97 | FICB with 0.25% Levobupivacaine 30ml (<50kg) or 40ml (>50kg) | Standard preoperative analgesia (Paracetamol 1g 6-hourly; Codeine 60mg 6-hourly; Opioid 10mg 2-hourly/prn) | SPA | ED | Trained anaesthesia trainees | Landmark technique | Pain score (0-10 VAS) at rest and on movement at 15mins, 2hrs & 8hrs | 1. Additional opioid consumption 2. Incidence of opioid overdose |
| Kassam et al.(18) 2018 | Non- RCT | 40 (20:20) |
No Range Given | FICB with 30-40ml 0.25% Levobupivacaine + traditional analgesia |
Traditional analgesia (regular paracetamol, mild opiates and oral morphine as required) | / | Not specified |
Orthopaedic & trauma registrar | Landmark technique | Pain score (0-10 NRS) at baseline, 1hr, 6hr & 12hr | 1. Morphine consumption |
| Ma et al.(21) 2018 |
RCT | 88 (44:44) |
No Range Given | FICB with 50ml 0.4% Ropivacaine THEN 0.2% Ropivacaine @ 5ml/hr via pump & catheter (if initial analgesic effect was insufficient, 5ml 0.2% Ropivacaine were added) |
Traditional analgesia: 50mg Tramadol 8-hourly PO + 500mg Paracetamol 8-hourly PO |
/ | Not specified |
Experienced anaesthetists | US guided technique |
Pain score (100- point VAS) at rest and on movement at: 1. Baseline 2. 1 hour 3. Before surgery 4. Day 1 post-op 5. Day 2 post-op |
1. Analgesia induced complications 2. Additional analgesia requirements 3. FICB complications |
| Pasquier et al.(19) 2019 | Double-Blind RCT | 30 (15:15) |
73 - 90 | FICB with 30ml 0.5% Bupivacaine + Epinephrine 1:200,000 |
5ml subcutaneous normal saline | 1.IV morphine (before intervention) 2. 1g Acitaminophen 6-hourly 3. IV morphine PRN for persistent pain |
ED | Experienced ED physician - not involved in study | Landmark technique | Pain score (NRS) at rest and on movement over first 45 mins | 1. Pain score at rest and on movement @ 4h, 8h, 12h, 24h 2. Total morphine consumption 3. LOS 4. Mortality |
| Wenn-berg et al.(20) 2019 |
Double-Blind RCT | 127 (66:61) |
65 - 99 | FICB with 30ml 2mg/ml Ropivacaine | FICB with 30ml saline | 1. Opioids (before intervention) | Ortho- paedic ward |
Trained orthopaedic surgeon | Landmark technique | Pain at rest and on movement at baseline, 15min, 2h & 6h using a modified VAS tool | 1. Additional IV morphine consumption 2. Additional paracetamol consumption 3. LOS |
| Hao et al.(22) 2019 |
RCT | 85 (43:42) |
No Range Given | FICB with 30ml 0.45% Ropivacaine + 0.25% Ropivacaine @ 6ml/hr via pump & catheter |
Continuous FICB using 0.9% saline | IM injection of 0.05mg fentanyl as required | ED | Anaesthetist | US guided technique |
Pain score (0-10 VAS) at baseline, 2hr, 4hr, morning of surgery, prior to anaesthesia | 1. Fentanyl consumption 2. Delirium 3. Post-op pain score |
| Garlich et al.(13) 2020 | Observational Study | 725 (92:633) |
No Range Given | FICB with 30-40ml 0.25% Bupivacaine + 1:200,000 Epinephrine or 10-20ml 0.2% Bupivacaine then continuous infusion of 0.2% Bupivacaine @ 6ml/h |
Historical control group who did not receive FICB | Not specified | ED & ward | Regional anaesthesia team | US guided technique |
Pain score (11-point VAS) | 1. Opioid consumption 2. Delirium 3. Opioid related side effects |
Table 1: Table highlighting the characteristics of the included studies.
Risk of Bias
All ten [13–22] studies were well-structured and were all deemed to have minimal risk of bias [11,12]. Assessments for risk of bias of RCTs (Table 2) and non-RCTs (Table 3) were performed and included in this review, highlighting the reasons for bias.
All ten [13–22] studies were well-structured and were all deemed to have minimal risk of bias [11,12]. Assessments for risk of bias of RCTs (Table 2) and non-RCTs (Table 3) were performed and included in this review, highlighting the reasons for bias.
| Author and Year | Selection Bias | Performance Bias | Detection Bias | Attrition Bias | Reporting Bias | Other Bias | |
| Random Sequence Generation | Allocation Concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete Outcome Data | Selective Reporting | ||
| Foss et al.(15) 2007 |
Low | Low | Low | Low | Low | Low | Low |
| Monzon et al.(14) 2010 | Low | Low | Unclear*1 | Low | Low | Low | Low |
| Ma et al.(21) 2018 |
Low | Low | High*2 | Unclear*3 | Low | Low | Low |
| Pasquier et al.(19) 2019 | Low | Low | Low | Low | Unclear*4 | Unclear*4 | Low |
| Wennberg et al.(20) 2019 | Low | Low | Low | Low | Low | Low | Low |
| Hao et al.(22) 2019 |
Low | Low | Unclear*5 | Low | Low | Low | Low |
| *1The nurse preparing the allocated treatment may have biased the patient/personnel. *2The patients were not blinded in view of ethical considerations. *3Lack of patient blinding may have biased outcome assessment. *4Data on adverse effects of FICB was omitted. *5Did not specify blinding of participants/personnel. |
|||||||
Table 2: Risk of Bias for RCTs using the Cochrane ROB-2(11) tool.
| Author and Year | Pre-Intervention | At Intervention | Post-Intervention | ||||
| Bias due to Confounding | Selection Bias | Bias in classification of interventions | Bias due to deviations from intended intervention | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of reported results | |
| Hanna et al.(16) 2014 | Low | Low | Low | Low | Low | Low | Low |
| Williams et al.(17) 2016 | Moderate*1 | Low | Low | Low | Low | Moderate*2 | Low |
| Kassam et al.(18) 2018 | Low | Low | Low | Low | Low | Moderate*3 | Low |
| Garlich et al.(13) 2020 | Low | Moderate*4 | Moderate*4 | Low | Low | Moderate*2 | Moderate*5 |
| *1The amount of analgesia given prior to FICB was not regulated. *2Knowledge of FICB may have influenced patients’ VAS for pain. *3All FICBs were administered by the author of the paper. *4Only the intervention group was recruited prospectively. *5Only 1 average pre-operative pain VAS was reported despite being checked every 4-6 hours. |
|||||||
Table 3: Risk of Bias for Non-RCTs using the Cochrane ROBINS-I(12) tool.
Four (14,15,19,20) of the six (14,15,19–22) included RCTs were double-blind trials, however, lack of blinding was observed to be a source of bias. This may have been due to the fact that the administration of an FICB is a rather conspicuous procedure, clearly differing from more traditional analgesia such as IV or oral treatment. Additionally, the administration of a placebo FICB raised some ethical concerns so its omission contributed to lack of blinding (21).
Contrary to RCTs, prospective non-RCTs were more susceptible to the Hawthorne effect as both patients and personnel were aware of the intervention. This was highlighted by the considerable level of bias in measurement of outcomes observed in three(13,17,18) of the four non-RCTs included.
Quality assessment of RCTs
Five (14,15,19–21) of the six RCTs addressed clearly focused questions with detailed PICO components, while Hao et al. (22) misrepresented PICO elements by suggesting post-operative delirium was the primary outcome instead of pain relief. Randomization methods were adequate in all six studies, with study groups starting similarly and receiving consistent care. Participant blinding was implemented in all studies except Ma et al. (21), who omitted blinding for ethical reasons, potentially introducing bias. Blinding of personnel was unclear in two studies, raising concerns about possible bias.
Five (14,15,19–21) of the six RCTs addressed clearly focused questions with detailed PICO components, while Hao et al. (22) misrepresented PICO elements by suggesting post-operative delirium was the primary outcome instead of pain relief. Randomization methods were adequate in all six studies, with study groups starting similarly and receiving consistent care. Participant blinding was implemented in all studies except Ma et al. (21), who omitted blinding for ethical reasons, potentially introducing bias. Blinding of personnel was unclear in two studies, raising concerns about possible bias.
All participants were accounted for at study conclusions, except for Hao et al. (22), where two patients were lost to follow-up due to catheter dislodgement. Treatment effects were precisely reported in three studies, and adverse effects were addressed in all but one (Pasquier et al. (19)). Despite these limitations, intervention effects were comprehensively reported in all studies.
Quality Assessment of Non-RCTs
The four non-RCT studies addressed clearly focused questions with detailed PICO components. Three(16–18) studies recruited participants prospectively and measured exposures accurately to minimize bias. In contrast, Garlich et al.(13) compared a historical cohort receiving conventional analgesia to a prospective cohort receiving FICB, potentially influencing participant recruitment and outcome assessment. Bias due to confounding was minimal in all studies, but inadequate documentation of pre-intervention medications and their impact on pain scores was a notable limitation. Three studies were comparable to well-performed RCTs, while Garlich et al.(13) provided strong evidence for evaluating FICB but fell short of RCT comparability.
The four non-RCT studies addressed clearly focused questions with detailed PICO components. Three(16–18) studies recruited participants prospectively and measured exposures accurately to minimize bias. In contrast, Garlich et al.(13) compared a historical cohort receiving conventional analgesia to a prospective cohort receiving FICB, potentially influencing participant recruitment and outcome assessment. Bias due to confounding was minimal in all studies, but inadequate documentation of pre-intervention medications and their impact on pain scores was a notable limitation. Three studies were comparable to well-performed RCTs, while Garlich et al.(13) provided strong evidence for evaluating FICB but fell short of RCT comparability.
Results of Individual Studies
Primary outcome
Seven studies (14–18,21,22) showed statistically significant reductions in pain scores after administration of an FICB. The study by Wennberg et al. (20) demonstrated significant reductions in pain scores in the FICB group but, after correcting for an imbalance in baseline pain scores, these differences were not retained. Two studies (13,19) did not demonstrate any significant difference in pain scores between intervention and control groups.
Seven studies (14–18,21,22) showed statistically significant reductions in pain scores after administration of an FICB. The study by Wennberg et al. (20) demonstrated significant reductions in pain scores in the FICB group but, after correcting for an imbalance in baseline pain scores, these differences were not retained. Two studies (13,19) did not demonstrate any significant difference in pain scores between intervention and control groups.
Seven(14–20) studies analysed the effects of a single-shot FICB. Of these, five(14–18) exhibited improved pain outcomes which were statistically significant while two(19,20) did not. Conversely, two studies(21,22) followed up the single-shot FICB with a continuous infusion of LA via a catheter. Ma et al.(21) showed statistically significant reductions in pain scores one hour after analgesia, both at rest and on passive movement. Hao et al.(22) also demonstrated significant reductions in pain scores in the first four hours after administration, persisting up until the time of anaesthesia. In their study, Garlich et al.(13) administered a single-shot FICB or an FICB plus an infusion of LA. They did not specify what proportion of patients received either of the two aforementioned options. Nonetheless, they reported no statistically significant differences in pain scores between the intervention and control groups.
Pain at rest was assessed in nine studies(13–15,17–22). Although four studies(13,14,18,22) did not explicitly specify if pain scores were taken at rest, there was no reason to assume otherwise. Only two studies(15,21) provided separate data for pain at rest and on movement. Foss et al.(15) noted a statistically significant improvement in VAS at rest at 60 (p=<0.01) and 180 (p=0.03) minutes and a significant improvement in VAS on movement at 180 minutes. Ma et al. (21) Had similar findings with significant reductions in pain scores at rest (p=0.023) and on passive movement (p=<0.05) at 60 minutes from block.
Timing of pain score assessment was a significant variable. All the included studies assessed pain scores within one hour of block administration. One study(13) only provided an average preoperative pain score despite taking measurements every 4-6 hours. Pain during the first hour after FICB administration was significantly reduced in four studies(14,15,18,21). Beyond the first hour, results varied. Foss et al.(15) demonstrated a significant reduction in pain score at three hours. Both studies by Hanna et al.(16) and Williams et al.(17) found significant reductions in pain scores between two and eight hours after the block was given with Kassam et al.(18) noting reduced pain scores at six and 12 hours. Conversely, despite noting lower pain scores after 15 minutes, Monzon et al.(14) did not observe any significantly reduced pain scores between two and eight hours. Only two studies(16,19) assessed the effect of FICB beyond 12 hours and neither reported significant reductions in pain scores.
Two studies(14,19) compared the efficacy of FICB to regular intravenous (IV) or oral NSAIDs respectively. In both instances, there was no significant difference in pain between the two groups other than a reduction in pain score at 15 minutes seen in the study by Monzon et al.(14).
Secondary outcomes
Eight studies assessed additional analgesia requirements. Six of these(13,15–18,22) demonstrated statistically significant (p=<0.05) reductions in additional analgesic requirements which were universally opiates. In the study by Foss et al.(15), no additional morphine was given to the FICB group whereas a median of 6mg of morphine was given to the control group (p=0.01). Pasquier et al.(19) observed no differences in additional morphine consumption between FICB and control groups with an average of 7mg and 8mg respectively (p=0.63). Findings were similar for Wennberg et al.(20) with no significant differences in additional morphine consumption at two (p=0.36) or six hours (p=0.37).
Eight studies assessed additional analgesia requirements. Six of these(13,15–18,22) demonstrated statistically significant (p=<0.05) reductions in additional analgesic requirements which were universally opiates. In the study by Foss et al.(15), no additional morphine was given to the FICB group whereas a median of 6mg of morphine was given to the control group (p=0.01). Pasquier et al.(19) observed no differences in additional morphine consumption between FICB and control groups with an average of 7mg and 8mg respectively (p=0.63). Findings were similar for Wennberg et al.(20) with no significant differences in additional morphine consumption at two (p=0.36) or six hours (p=0.37).
Seven studies(14–18,21,22) reported on the safety profile of FICB and no serious complications were reported. Foss et al.(15) reported that the control group were more sedated and had a tendency towards lower oxygen saturation. Monzon et al(14) noted four instances of delirium in the IV NSAID group whereas no episodes occurred in the FICB group. The incidence of opioid overdose was assessed in one study(17) whereby the control group receiving standard care showed a significantly more frequent occurrence (7.2% vs 0%; p=0.001). One study(13) could not find any statistical difference in opioid related side effects between the two groups.
Only two studies assessed the incidence of delirium, with conflicting results. Hao et al.(22) report a significantly decreased incidence of post-operative delirium in the FICB group (13.9% vs 35.7%; p=0.018) whereas Garlich et al.(13) could not demonstrate a significant difference in the incidence of delirium between the two groups. A summary of the outcomes of each study is provided in table 4.
| Author and year | Primary Outcome: Pain Assessment | Secondary Outcome: Additional Analgesic requirements |
| Foss et al.(15) 2007 | +At rest: Pain improvement in FICB group at 60 (p=<0.01) & 180 (p=0.03) minutes compared to control group +On 15 degrees movement: Pain improvement in FICB group at 180 (p=0.04) minutes compared to control group |
+No additional morphine was administered to FICB group vs a median 6mg in the morphine group (p=0.01) |
| Monzon et al.(14) 2010 | +FICB was more effective at 15 minutes (p=0.001). Pain relief was similar between 2 & 8 hours | Not studied |
| Hanna et al.(16) 2014 | +Reduction in pain score in FICB group at 2 hours (p=0.03) which continued to 8 hours (p=0.01) =Pain scores at 16 and 24 hours reduced by half but not significant +Time to initial analgesia was reduced by 25 minutes in FICB group vs 40 minutes in control group (p=0.04) |
+Systemic analgesia requirements were reduced in the FICB group within the first 24 hours with a median 3.5 doses vs 1.5 doses in the control group (no p-value given) |
| Williams et al. (17) 2016 | +Pain score was lower in the FICB group vs no FICB group (p=0.001) | +FICB group had significant reduction in additional opioid doses from 6.2 to 2 doses (p=0.001) |
| Kasssam et al. (18) 2018 | +Reductions in pain score in FICB group at 1, 6 & 12 hours (p=0,0,0) when compared to control group | +FICB group had significant reduction in average additional morphine use: 11mg vs 60.5mg (p=0.05) |
| Ma et al.(21) 2018 | +Reduction in pain score at rest 1 hour after analgesia in FICB vs control group (p=0.023) +Reduction in pain score on passive movement 1 hour after analgesia in FICB vs control group (p=<0.05) |
Not studied |
| Pasquier et al. (19) 2019 | =No reduction in pain score was observed in patients receiving FICB | =Total morphine consumption was similar in both groups: 7mg in FICB group vs 8mg in the sham injection group (p=0.63) |
| Wennberg et al. (20) 2019 | =Significant reduction in pain score between FICB and control groups. However, after adjusting for imbalance at baseline, this difference was not retained. |
=No significant difference in morphine consumption between both groups at 2 hours (2.2mg vs 2.3mg; p=0.36) or 6 hours (3.1mg vs 3.4mg; p=0.37) |
| Hao et al.(22) 2019 | +SS reduction in VAS pain score in FICB group when compared to control group. (p N/A) | +Consumption of fentanyl was significantly less in the FICB group vs control group: 0.08 vs 0.28 (p=0.037) |
| Garlich et al.(13) 2020 | =No difference in preoperative pain scores was observed | +FICB group consumed 40% less morphine milliequivalent (p=0.007) |
Table 4: Table highlighting the primary outcome (pain assessment) and secondary outcome (additional analgesic requirements) for all studies.
+ Significant benefit; = Similar or no significant difference.
+ Significant benefit; = Similar or no significant difference.
Discussion
Pain Relief
Hip fractures are highly painful injuries, and the most effective analgesia is surgical repair and fixation, ideally performed within 48 hours, as this reduces 1-year mortality by 20% (Klestil et al.)(23). Optimal recovery requires both timely surgery and adequate pain management from the emergency room until the procedure. The NICE guidelines recommend using nerve blocks alongside paracetamol and opioids for additional pain relief while reducing opioid-related side effects. However, the guidelines do not specify which nerve block technique to use, leading to variability in block characteristics (e.g., drug type, dosage, single-shot vs. continuous infusion) and pain assessment timing. This lack of specificity reflects the absence of a clear consensus in the literature on the best method.
Hip fractures are highly painful injuries, and the most effective analgesia is surgical repair and fixation, ideally performed within 48 hours, as this reduces 1-year mortality by 20% (Klestil et al.)(23). Optimal recovery requires both timely surgery and adequate pain management from the emergency room until the procedure. The NICE guidelines recommend using nerve blocks alongside paracetamol and opioids for additional pain relief while reducing opioid-related side effects. However, the guidelines do not specify which nerve block technique to use, leading to variability in block characteristics (e.g., drug type, dosage, single-shot vs. continuous infusion) and pain assessment timing. This lack of specificity reflects the absence of a clear consensus in the literature on the best method.
Studies(21,22) on continuous infusion of local anesthetics (LA) for pain relief after hip fractures lacked data beyond four hours, making it unclear if this method offers benefits over single-shot FICB. In contrast, studies(17,18) on single-shot FICB demonstrated improved pain scores at 8 and 12 hours, with one study(16) reporting reduced pain scores at 16 and 24 hours, though not statistically significant. These findings suggest that a single-shot FICB with a long-acting LA may provide effective analgesia lasting up to 12 hours.
FICB was directly compared to several regimens of opioids namely, morphine(15), tramadol(21) and fentanyl(22). In all instances, significant improvement in pain was observed in the FICB groups, both at rest and on movement, suggesting that FICB may provide superior analgesia to opioids. FICB was also compared to NSAIDs in one study by Monzon et al.(14). They demonstrated that FICB provided more effective analgesia within the first 15 minutes but between 2-8 hours, pain relief was similar in the FICB group to the IV NSAID group. This data suggests that FICB is similarly effective at providing pain relief in hip fractures as NSAIDs, however, the adverse effects of NSAIDs, including an increased risk of fracture(24), are well documented in the literature and thus, FICB may be a more appealing approach.
Of note, none of the included studies referred to any non-pharmacological means of analgesia, such as traction or urinary catheterisation (to decrease need for mobilisation) that may or may not have been used and this may have caused some bias. These findings are in keeping with several other systematic reviews in the literature by Steenberg et al(25)., Wan et al.(26), and Pinson et al.(8)
Minimisation of opioid consumption
Despite their several side-effects, opioids remain the mainstay of pain management in hip fracture patients. Indeed, the eight studies (13,14,16–20,22) in this review assessing additional analgesia requirements all measured opioid consumption. In the elderly, physiological changes such as increased adipose tissue can lead to delayed elimination of drugs, including opioids, and thus their adverse effects can be exaggerated (27).
Despite their several side-effects, opioids remain the mainstay of pain management in hip fracture patients. Indeed, the eight studies (13,14,16–20,22) in this review assessing additional analgesia requirements all measured opioid consumption. In the elderly, physiological changes such as increased adipose tissue can lead to delayed elimination of drugs, including opioids, and thus their adverse effects can be exaggerated (27).
At least four other reviews(5,25,26,28) have reported that FICB significantly reduced the need for additional opioid administration. This is in keeping with the findings of this review where six(13,15–18,22) out of eight studies reported decreased opioid requirements. Contrarywise, Pasquier et al.(19) noted that total morphine consumption was similar in both intervention and control groups but one should note that both groups received only small doses of morphine and that all patients were given regular doses of acetaminophen. Wennberg et al.(20) did not find a significant difference in morphine consumption although they administered a low dose of ropivacaine in their blocks which may have affected their results. Although the studies in this review each have their own limitations, the data is very compelling and serves a strong indicator that FICB is very effective in minimising additional opioid consumption as well as any resultant complications.
Incidence of Delirium
Delirium is a common complication in elderly hip fracture patients, particularly after surgery. Major risk factors include prior cognitive impairment, male gender, advanced age, and morphine usage, with morphine increasing the risk of delirium by over threefold according to a systematic review by Yang et al.(29), though the data showed significant variability. Cognitive screening has been suggested as part of standard care for these patients. The impact of FICB on delirium is unclear, with one study(29) showing reduced delirium incidence while another(13) did not, potentially due to differences in patient selection (e.g., inclusion of patients with dementia). Regardless, patients with dementia should still receive appropriate analgesia, including FICB, if deemed effective.
Delirium is a common complication in elderly hip fracture patients, particularly after surgery. Major risk factors include prior cognitive impairment, male gender, advanced age, and morphine usage, with morphine increasing the risk of delirium by over threefold according to a systematic review by Yang et al.(29), though the data showed significant variability. Cognitive screening has been suggested as part of standard care for these patients. The impact of FICB on delirium is unclear, with one study(29) showing reduced delirium incidence while another(13) did not, potentially due to differences in patient selection (e.g., inclusion of patients with dementia). Regardless, patients with dementia should still receive appropriate analgesia, including FICB, if deemed effective.
Safety and adverse effects of FICB
The safety of FICB has been widely documented throughout the literature and it is universally regarded as a safe procedure(14,16,17,26). This review has corroborated this as none of the included studies reported any serious adverse outcomes. Of note, the personnel administering the block ranged from regional anaesthesia team members to junior anaesthetists with basic training. The wide variety of staff performing blocks indicates that FICB is a simple and safe procedure that may be administered with minimal training and sparse complications. Conversely, one could speculate that FICB may be more effective if administered by anaesthetists rather than junior staff.
The safety of FICB has been widely documented throughout the literature and it is universally regarded as a safe procedure(14,16,17,26). This review has corroborated this as none of the included studies reported any serious adverse outcomes. Of note, the personnel administering the block ranged from regional anaesthesia team members to junior anaesthetists with basic training. The wide variety of staff performing blocks indicates that FICB is a simple and safe procedure that may be administered with minimal training and sparse complications. Conversely, one could speculate that FICB may be more effective if administered by anaesthetists rather than junior staff.
Conclusion
This review demonstrates that FICB provides similar, and sometimes superior, pain relief for hip fractures compared to traditional systemic analgesia, without serious adverse outcomes. FICB is best used as part of a tailored pain management plan rather than in isolation. A key benefit of FICB is its ability to reduce opioid consumption, which is beneficial for patient outcomes and mortality. FICB is a safe, easy-to-perform procedure that can be quickly administered in emergency settings with readily available equipment. It offers comparable or superior analgesia to opioids and NSAIDs, with a better safety profile. Moreover, FICB reduces the need for additional analgesia, thereby minimizing drug-related side effects. Although the role of FICB in reducing delirium remains unclear, its potential benefit, particularly by reducing opioid use, is promising. Future research should focus on standardized treatment protocols and randomized controlled trials (RCTs) to compare single-shot versus continuous FICB for longer-lasting pain relief.
References
- Marks R. (2010). Hip fracture epidemiological trends, outcomes, and risk factors, 1970-2009.. Int J Gen Med. Apr 8;3:1–17.
- Cooper C, Campion G, Melton LJ. (1992). Hip fractures in the elderly: A world-wide projection. Osteoporos Int. Nov;2(6):285–9.
- Cummings SR, Melton LJ. (2002). Epidemiology and outcomes of osteoporotic fractures. The Lancet. May;359(9319):1761–7.
- Rapp K, Büchele G, Dreinhöfer K, Bücking B, Becker C, Benzinger P. (2019). Epidemiology of hip fractures: Systematic literature review of German data and an overview of the international literature. Z Für Gerontol Geriatr. Feb;52(1):10–6.
- Ritcey B, Pageau P, Woo MY, Perry JJ. (2016). Regional Nerve Blocks For Hip and Femoral Neck Fractures in the Emergency Department: A Systematic Review. CJEM. Jan;18(1):37–47.
- Recommendations | Hip fracture: management | Guidance | NICE [Internet]. NICE; [cited 2021 Mar 18]. Available from: https://www.nice.org.uk/guidance/cg124/chapter/Recommendations#analgesia
- Dalens B, Vanneuville G, Tanguy A. (1989). Comparison of the fascia iliaca compartment block with the 3-in-1 block in children. Anesth Analg. Dec;69(6):705–13.
- Pinson S. (2015). Fascia Iliaca (FICB) block in the emergency department for adults with neck of femur fractures: A review of the literature. Int Emerg Nurs. Oct;23(4):323–8.
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JPA, et al. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. Dec 4;339(jul21 1): b2700–b2700.
- Critical Appraisal Skills Programme. CASP Checklists [Internet]. 2019. Available from: https://casp-uk.net/casp-tools-checklists/
- Sterne JAC, Savovic J, Page M, Elbers R, Blencowe N, Boutron I, et al. (2019). RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ.;366(I4898).
- Sterne JAC, Hernan M, Reeves B, Savovic J, Berkman N, Viswanathan M, et al. (2016). ROBINS-I: a tool for assessing risk of bias in non-randomized studies of interventions. BMJ. 355(I4919).
- Garlich JM, Pujari A, Moak Z, Debbi E, Yalamanchili R, Stephenson S, et al. (2020). Pain Management with Early Regional Anesthesia in Geriatric Hip Fracture Patients. J Am Geriatr Soc. Sep;68(9): 2043–50.
- Monzón DG, Vazquez J, Jauregui JR, Iserson KV. (2010). Pain treatment in post-traumatic hip fracture in the elderly: regional block vs. systemic non-steroidal analgesics. Int J Emerg Med. Dec;3(4): 321–5.
- Foss NB, Virkelyst C. (2007). Fascia Iliaca Compartment Blockade for Acute Pain Control in Hip Fracture Patients.;106(4):6.
- Hanna L, Gulati A, Graham A. (2014). The Role of Fascia Iliaca Blocks in Hip Fractures: A Prospective Case-Control Study and Feasibility Assessment of a Junior-Doctor-Delivered Service. ISRN Orthop. Mar 4; 2014:1–5.
- Williams H, Paringe V, Shenoy S, Michaels P, Ramesh B. (2016). Standard Preoperative Analgesia with or without Fascia Iliaca Compartment Block for Femoral Neck Fractures. J Orthop Surg. Apr;24(1): 31–5.
- Kassam AAM, Gough AT, Davies J, Yarlagadda R. (2018). Can we reduce morphine use in elderly, proximal femoral fracture patients using a fascia iliac block? Geriatr Nur (Lond). Jan;39(1):84–7.
- Pasquier M, Taffé P, Hugli O, Borens O, Kirkham KR, Albrecht E. (2019). Fascia iliaca block in the emergency department for hip fracture: a randomized, controlled, double-blind trial. BMC Geriatr. Dec;19(1): 180.
- Wennberg P, Norlin R, Herlitz J, Sarenmalm EK, Möller M. (2019). Pre-operative pain management with nerve block in patients with hip fractures: a randomized, controlled trial. Int J Orthop Trauma Nurs. May; 33:35–43.
- Ma Y, Wu J, Xue J, Lan F, Wang T. (2018). Ultrasound-guided continuous fascia iliaca compartment block for pre-operative pain control in very elderly patients with hip fracture: A randomized controlled trial. Exp Ther Med [Internet]. Jul 6 [cited 2021 Feb 6]; Available from: http://www.spandidos-publications.com/10.3892/etm.2018.6417
- Hao J, Dong B, Zhang J, Luo Z. (2019). Pre-emptive analgesia with continuous fascia iliaca compartment block reduces postoperative delirium in elderly patients with hip fracture. A randomized controlled trial. Saudi Med J. Sep 9;40(9): 901–6.
- Klestil T, Röder C, Stotter C, Winkler B, Nehrer S, Lutz M, et al. (2018). Impact of timing of surgery in elderly hip fracture patients: a systematic review and meta-analysis. Sci Rep. Dec;8(1):13933.
- Mortensen SJ, Mohamadi A, Wright CL, Chan JJ, Weaver MJ, von Keudell A, et al. (2020). Medications as a Risk Factor for Fragility Hip Fractures: A Systematic Review and Meta-analysis. Calcif Tissue Int. 107(1): 1–9.
- Steenberg J, Møller AM. (2018). Systematic review of the effects of fascia iliaca compartment block on hip fracture patients before operation. Br J Anaesth. Jun;120(6):1368–80.
- Wan H yang, Li S yi, Ji W, Yu B, Jiang N. (2020). Fascia Iliaca Compartment Block for Perioperative Pain Management of Geriatric Patients with Hip Fractures: A Systematic Review of Randomized Controlled Trials. Arai YC, editor. Pain Res Manag. Nov 25; 2020:1–12.
- Chau DL, Walker V, Pai L, Cho LM. (2008). Opiates and elderly: use and side effects. Clin Interv Aging. 3(2): 273–8.
- Chesters A, Atkinson P. (2014). Fascia iliaca block for pain relief from proximal femoral fracture in the emergency department: a review of the literature. Emerg Med J. Oct;31(e1): e84–7.
- Yang Y, Zhao X, Dong T, Yang Z, Zhang Q, Zhang Y. (2017). Risk factors for postoperative delirium following hip fracture repair in elderly patients: a systematic review and meta-analysis. Aging Clin Exp Res. Apr;29(2): 115–26.
Citation: Isaac Balzan and Christabel Balzan. (2025). The use of preoperative Fascia Iliaca Compartment Block (FICB) as an effective form of analgesia in adult patients with hip fractures: A systematic review. Journal of Orthopaedic and Trauma Care 6(1).
Copyright: © 2025 Isaac Balzan. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.