Research Article
Volume 5 Issue 1 - 2024
Influence of the Ketogenic Diet on Mandibular Growth and its Potential Impact in Facial Development. A Cephalometric Study in Growing Rats.
Department of Histology and Embriology, Faculty of Dentistry. National University of Tucumán
*Corresponding Author: Garat Juan Abel, Av. Benjamín Araoz 800. 4000. San Miguel de Tucumán. Argentina.
Received: April 25, 2024; Published: May 13, 2024
Abstract
Objective: To evaluate the development of mandibular units and its likely impact on facial development, and on the space required for alignment of the teeth without crowding in a model of ketogenic diet in growing rats.
Methods: Wistar rats weaned at 21 days were assigned to one of the following groups: control (fed a regular hard diet) and experimental (fed a ketogenic hard diet). After 30 days ketonemia was determined and animals were euthanized. The amount of food consumed and the weight of animals were recorded. After resection of the mandibles, they were hemisected and fixed in 10% formalin. Soft tissue was removed. Metallic landmarks were placed in mental and mandibular foramens of one hemimandible of each rat. Hemimandibles were radiographed and cephalometric analysis was performed. Data obtained were analyzed with Student "t" Test.
Results: Food consumed was lower in the experimental group. Body weight was in Controls: 179±1 g and 128 ± 11 g in experimental group, p<0,05. Ketonemia was in controls: 0,29±0,042mmol/l and in Experimentals 1,89±0,7 mmol/l, p<0,05. Lengths of the mandible as a whole, the condylar process, the convexity of the angular process and the alveolar process of the lower first molar were significantly lower in the experimental group. No significant differences were observed in the ratio between the mesiodistal dimensions of the molars and the length of the mandibular body.
Conclusions: These results suggest that the ketogenic diet negatively alters the development of the condylar process and the angle of the mandible, promoting a dolichofacial growth pattern.
Keywords: Ketogenic diet; Mandibular growth; Facial development
Main Points
- The ketogenic diet is used successfully for the treatment of refractory epilepsy in growing children. Its effects on long bones have been widely studied. However, its effects on the jaw and its possible implications on facial development have not been studied until now.
- During normal facial growth, the mandibular symphysis moves down and forward along Rickett’s facial axis. This movement is the result of vertical and saggital growth vectors. But it is the vertical growth vector that has a crucial effect on the saggital (anteroposterior) growth direction of the mandibular symphysis. This growth pattern results from the descent of the glenoid fossae and, essentially, from the increase in condyle length.
- In this study the mandibular units that exhibited a decrease in size compared to controls are structures that grow and develop by endochondral ossification such as the mandibular condyle. These results suggest that the ketogenic diet negatively alters the development of the condylar process and the angle of the mandible, promoting a dolichofacial growth pattern.
Introduction
Facial development is a highly complex process and may be vulnerable to endogenous (genetic) and exogenous (environmental) factors. Although the shape and size of the head, face and dental arches are determined by genetic factors [1], craneo-facial development is affected by external factors during growth. Studies in the literature have reported certain habits, trauma, and premature extraction of deciduous teeth to alter normal growth direction and to reduce arch length, causing crowding [2]. In addition, there are other factors, such as nutritional alterations, that may appear during growth and affect bone development, causing marked variations in bone shape and size. During fetal and postnatal growth, there is a close relation between overall nutritional status and longitudinal bone growth. [3].
It is well documented that Low-carbohydrate, high-fat (LC–HF) diets are commonly promoted for weight loss around the globe, particularly “Atkins-style” diets which encourage the consumption of fat and protein and limit carbohydrate intake [4]. Further restriction of protein content of LC–HF diets results in a ketogenic diet, which is an established treatment option for epilepsy in children [5, 6]. Despite the established effectiveness in controlling seizures, the effect of ketogenic diets reported a number of unfavourable metabolic effects such as an increase in adiposity and glucose intolerance [7–9] with higher serum leptin concentrations and a concurrent loss of lean body mass in human and animal studies [10, 11]. In addition, diets with an altered relative abundance of fat, protein and carbohydrates affect bone metabolism and alters skeletal system with alterations in which could be loss of bone mineral density, bone volume, and trabecular number and biomechanical properties in long bones [12].
However, the effects of the ketogenic diet on the growth, shape and displacement of the mandible during facial development as well as in the ratio between mesiodistal tooth size to dental arch length have not been evaluated to date.
Given the wide use of well-defined, ketogenic LC–HF diets as a treatment option for epilepsy in growing children [5, 6] it is evident that the mandible develops under the negative influence of this nutritional alteration. It can therefore be inferred that ketogenic diet may affect normal facial develop. Based on the above, the aim of the present experimental work was to assess the influence of ketogenic diet on the dimensions of differents mandibular units, and the implications that these ketogenic diet-related alterations would have in the direction of mandibular displacement during facial development by means of cephalometric evaluation.
Materials and Methods
The experimental procedures were carried out in accordance with ARRIVE guidelines. The National Institutes of Health Guidelines for the Care and Use of Laboratory Animals (NHI publication 85-23 Rev. 1985) were observed.
Twenty newly born Wistar rats were used. The animals were weaned at the age of 21 days and assigned to one of the following groups: control group (n=10) fed a regular hard diet ad libitum (Table1), and experimental group (n=10) fed a ketogenic hard diet ad libitum (Table 1) [13]. Body weight and food consumed by the animals of both groups was recorded throughout. Five weeks after the onset of the experiment (rats aged 8 weeks) blood samples were obtained by cutting tail veins and ketonemia was determined by colorimetric methods. The animals were euthanized following international protocols. Once sedated with an intraperitoneal injection of 0.25 ml of a 5% ketamine solution (Holliday-Scott SA) at a dose of 40 mg/kg body weight and 0.5 ml of a 1% acepromazine solution (Acedan, Holliday-Scott SA) at a dose of 160 mg/kg body weight, euthanasia was performed with an I.P injection of 0.20 ml of 4% sodium pentobarbital and 0.5% sodium diphenylhydantoin (Euthany, Brouwer SA) at a dose of 160 mg/kg of weight. The mandibles were then carefully dissected and fixed in 10% formalin, after which the soft tissue was removed. In order to perform the radiographic study, the mandibles were hemisected at the symphysis. Metallic landmarks consisting of L shaped 0.2mm steel ligature wires [14] were placed in the mental and the mandibular foramens of one hemimandible of each rat (Figure 1). The marked hemimandibles were positioned laterally on the film next to a 10mm long wire and radiographed using standard X-ray equipment at 70Kv and 8mA, 0.3 sec exposure time; the focus to film distance was 40cm. Reference points were marked on paper tracings of the projected image of the radiographs (X7 magnification) in order to perform the cephalometric measurements (Figure 1). Cephalometric reference points identifications, tracings, and measurements were conducted by the same author. Linear mandibular dimensions measured on the lateral roentgenograph of the mandible are shown in table 2 [14]. The projected image of the wire served as a scale and the measurements were calibrated according to the image of the standard length of wire and expressed in millimeters. The relationship between molar mesiodistal width and the length of the mandibular corpus was calculated using the ET/RS ratio (Figure 1, Table 2).
Statistical Analysis
Data were analyzed using SPSS software for Windows (version 22.0, IBM Corp, Armonk, NY, USA). The normal distribution of continuous variables was assessed using the Shapiro-Wilk test. Student “t” test was used to compare linear mandibular dimensions and in ET/RS ratio between control and experimental groups. Differences were considered significant with p<0.05.
Data were analyzed using SPSS software for Windows (version 22.0, IBM Corp, Armonk, NY, USA). The normal distribution of continuous variables was assessed using the Shapiro-Wilk test. Student “t” test was used to compare linear mandibular dimensions and in ET/RS ratio between control and experimental groups. Differences were considered significant with p<0.05.
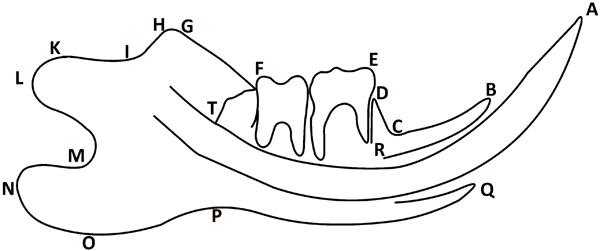
Figure 1: Reference points defined on the lateral roentgenograph of the mandible, according to Kiliaridis S (14). A: The most prominent point of the incisal edge of lower incisors. B: Lingual alveolar crest of lower incisors. C: Intersection between the lower incisor alveolar process and lower first molar alveolar process. D: Alveolar process mesial crest of the lower first molar. E: Mesial buccal cusp of the lower first molar. F: Intersection between the occlusal plane and the anterior border of the mandibular ramus. G: The most anterior and superior point of the coronoid process. H: The most posterior and superior point of the coronoid process. I: The most anterior point of the mandibular notch. J: The most inferior point of the mandibular notch. K: Fovea pterigoidea. L: The most posterior point of the condylar process. M: The deepest point of the posterior notch in the ramus of the mandibles. N: The most posterior point of the angular process of the mandible. O: The most inferior point of the lower border of the angular process. P: The deepest point of the inferior border of the mandible. Q: Vestibular alveolar crest of lower incisors. R: Position of markers in mental foramen. S: Position of markers in mandibular foramen. T: The most posterior point of the distal face of the third molar.
| Projected (per 100 g) | Standard diet | Ketogenic diet |
| Energy | 1338 kJ | 1748 kJ |
| Protein | 14,5 gr | 6,1 gr |
| Fat | 4 gr | 37,1 g |
| Carbohydrates | 55,5gr | 3,1gr |
| Dietary Fiber | 4,5gr | 27,5gr |
| Natrium | 130mg | 144mg |
| Calcium | 720mg | 117mg |
| Phosphorus | 600mg | 81mg |
| Vitamin D | 2,5 µg | 0,03 µg |
Displayed nutrients of ketogenic diet is less than 100g due to the loss in manufacture.
Table 1: Comparison between standard diet and ketogenic diet.
Table 1: Comparison between standard diet and ketogenic diet.
| B-N | Total mandibular length. |
| R-S | Chord of the mandibular canal representing the length of the mandibular corpus. |
| B-R | Length of the incisal process |
| K-L | Length of the condylar head. |
| S-K | Length of the condylar process. |
| P-N | Length of the angular process. |
| E-T | Total mesio-distal width of the 1st, 2nd, and 3rd molars. |
| O-PN | Angular process to mandibular body vertical relationship. The shortest distance between point O and line PN. |
| O-RS | Convexity of the angular process. The shortest distance between point O and line RS. |
| C-D | Height of the lower 1st molar alveolar process. |
| ET/RS | Ratio between the molars mesiodistal width and the chord of the mandibular canal. |
The variables were defined between two points or between a point and a line. (According to Kiliaridis S (14)).
Table 2: Variables measured on the lateral roentgenograph of the mandible.
Table 2: Variables measured on the lateral roentgenograph of the mandible.
Results
In the experimental group body weight and the amount of food consumed were lower, while ketonemia was significantly higher. Figures 2 and 3.
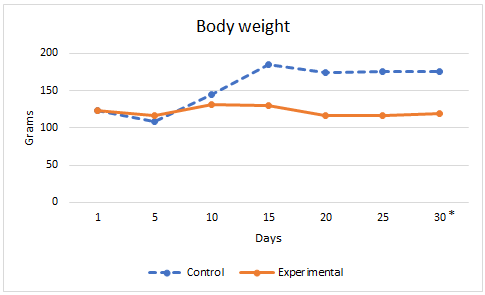
Figure 2: Arithmetic mean of body weight in control and experimental groups. *p<0,05. Student’s t-test.
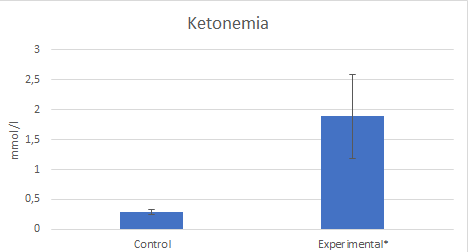
Figure 3: Arithmetic mean and standard deviation of ketonemia in control and experimental groups. *p<0,05. Student’s t-test.
The cephalometric study showed that the lengths of the mandible, the condylar process, the convexity of the angular process and the alveolar process of the lower first molar were significantly lower in the experimental group. No significant differences were observed in the ratio between the mesiodistal dimensions of the molars and the length of the mandibular corpus. Table 3.
| Control: Standar Diet | Experimental: Ketogenic Diet | p value | |
| B-N | 21,8 ± 1 | 19,7 ± 0,8 | < 0,05* |
| R-S | 11,9 ± 1 | 11,8 ± 1 | > 0,05 |
| B-R | 5,6 ± 0,4 | 4,6 ± 0,3 | > 0,05 |
| K-L | 1,8 ± 0,1 | 1,6 ± 0,6 | > 0,05 |
| S-K | 5,2 ± 0,1 | 4,6 ± 0,4 | < 0,05* |
| P-N | 8,8 ± 1 | 7,5 ± 0,6 | > 0,05 |
| E-T | 5,4 ± 0,5 | 5,2 ± 0,4 | > 0,05 |
| O-PN | 2,04 ± 0,6 | 1,54 ± 0,4 | > 0,05 |
| O-RS | 6,9 ± 0,02 | 5,6 ± 0,3 | < 0,05* |
| C-D | 2,1 ± 0,3 | 1,2 ± 1 | < 0,05* |
| ET/RS | 0,45 ± 0,007 | 0,46 ± 0,6 | > 0,05 |
X ± SD.
Measurements in millimeters
*Student’s t test.
Table 3: Cephalometric determinations.
Measurements in millimeters
*Student’s t test.
Table 3: Cephalometric determinations.
Discussion
The present study shows that ketogenic diet in growing rats alters the length of the mandible as a whole (B-N), the condylar process (S-K), the convexity of the angular process (O-RS) and the alveolar process of first molar height (C-D). However, the diet used in this study did not affect the ratio between total molar mesiodistal width and the length of the mandibular corpus (ET/RS).
Several authors have reported that ketogenic diet has adverse reactions and impact on long bones growth and development in patients with intractable epilepsy [12, 15, 16]. However, there are no publications that report the effects of the ketogenic diet on the mandible. In the present work we address this aspect and try to infer whether these alterations could alter the normal pattern of facial growth and on the ratio between the space available in the arch and the space required for alignment of the teeth without crowding. According to the results obtained in the present study, ketogenic diet affected total mandibular length (B-N). However, no significant differences in mandibular conduct chord length (R-S) and incisal process length (B-R) were observed between groups, showing that the diet failed to induce alterations in the frontal aspect of the mandible. (B-N) is a parameter that is clearly influenced by the values ??of (P-N) and (B-R). Individually these last two parameters did not show significant differences between both groups studied, although the arithmetic means of their absolute values ??were lower in the experimental group. In this context, it is likely that the sum of (P-N) and (B-R) is responsible for the significant differences recorded in (B-N). In addition, it is possible that the differences in values corresponding to the frontal aspect of the mandible failed to reach statistical significance due to the presence of continuously growing incisors, which were probably not affected by the diet.
In this study the mandibular units that exhibited a decrease in size compared to controls are structures that grow and develop by endochondral ossification. It is well documented that this type of ossification is particularly sensitive to a Ketogenic diet in patients with epilepsy [4, 10, 12].
The condyle is the main growth center of the mandible [17, 18] and plays an essential role in the development of the facial type. It is well documented that during normal facial growth, the mandibular symphysis moves down and forward with respect to the other facial structures along Rickett’s facial axis [16, 18]. This movement is the result of vertical and saggital growth vectors. But it is the vertical growth vector that has a crucial effect on the saggital (anteroposterior) growth direction of the mandibular symphysis. This growth pattern results from the descent of the glenoid fossae and, essentially, from the increase in condyle length [17, 18].
The development of both these components compensates simultaneously for the vertical growth of the upper jaw and the upper alveolar process, and of the lower alveolar process [17].
According to the results obtained in this study, it could be posited that the lack of development of the condylar process in growing children fed a ketogenic diet could alter the normal pattern of facial growth. The significant decrease in the size of the condylar process might favor the loss of vertical control. In this context, the spatial position of the mandible with respect to the base of the skull would be altered, resulting in ideal conditions for a growth pattern characterized by a downward and backward displacement of the mandibular symphysis and a posterior rotation of the mandible. This situation could result in a dolichofacial growth pattern. This type of alteration in the growth pattern caused by ketogenic diet could also cause an increase in the angle to the base of the skull and a downward inclination of the occlusal plane. Under these conditions it would be plausible to posit that the lower incisors would drift upward and forward to compensate the posterior rotation of the mandible, also determining a marked occlusal curve. Although this study showed that the height of the alveolar process of the lower first molar (C-D) was significantly lower in the experimental group, it is likely that the lack of condylar growth would result in premature contact, so that an anterior open bite could be a finding in this type of nutritional alterations. Furthermore, the significant differences described herein in the convexity of the angular process (ORS), reveal a lack of development of the angular process. It is well known that the lack of development of the angular process clinically involves an increase in the value of the gonial angle. These conditions lead to the logical inclination of the occlusal plane associated to a tendency to posterior displacement of the mandibular symphysis. This pattern of mandible development is characteristic of individuals with a dolichofacial growth pattern [17].
The results obtained in this study show a decrease in (B-N) values, indicating an alteration in the anteroposterior growth of the mandible. Given that the mesiodistal length of the molar crowns (E-T) showed no alterations, since they were fully developed by the onset of the experiment, it would be reasonable to predict that this situation might result in a discrepancy between the space required for proper alignment of the teeth, without overcrowding, and the space available in the mandibular arch. In the present investigation, this relationship was analyzed considering the ratio of the total mesiodistal length from the first to the third molar (E-T), as an indicator of the required space, to the mandibular canal chord length (R-S), as an indicator of the space available in the arch. No significant differences were observed between the control and the experimental groups when comparing both parameters separately as well as the ratio between these (ET/RS). Given that ketogenic does not affect the length of the mandibular corpus, it is improbable that dental crowding should occur in the rat, and overcrowding could not be a frequent finding in this type of nutritional deficiency. However, extrapolation of these results to humans should be done with caution. Mandibular canal chord length was used as a parameter of mandibular corpus growth based on reports in the literature showing its suitability for evaluation of alterations in mandibular dimensions and shape [20].
The results found in this study suggest that the ketogenic diet can affect the development of different mandibular units and consequently facial development. Given that it is a therapeutic diet that gives excellent results for the control of seizures in children with epilepsy, it cannot be suspended. In this context, consulting an orthodontist is recommended in this type of patient with the aim of using interceptive orthodontics to try to prevent or minimize the negative effects of the ketogenic diet on facial development.
References
- Martín AE, del R Pani M, Holgado NR, López Miranda LI, Meheris HE, Garat JA. (2014). Facial development disorders due to inhibition to endochondral ossification of mandibular condyle process caused by malnutrition. Angle Orthod. 84(3): 473-478.
- Zhao J, Jin H, Li X, Qin X. (2023). Dental arch spatial changes after premature loss of first primary molars: a systematic review and meta-analysis of split-mouth studies. BMC Oral Health. 23(1): 430.
- Masztalerz-Kozubek D, Zielinska-Pukos MA, Hamulka J. (2021). Maternal Diet, Nutritional Status, and Birth-Related Factors Influencing Offspring's Bone Mineral Density: A Narrative Review of Observational, Cohort, and Randomized Controlled Trials. Nutrients. 13(7): 2302.
- Merlotti D, Cosso R, Eller-Vainicher C, Vescini F, Chiodini I, Gennari L, Falchetti A. (2021). Energy Metabolism and Ketogenic Diets: What about the Skeletal Health? A Narrative Review and a Prospective Vision for Planning Clinical Trials on this Issue. Int J Mol Sci. 22(1): 435.
- Ulamek-Koziol M, Czuczwar SJ, Januszewski S, Pluta R. (2019). Ketogenic Diet and Epilepsy. Nutrients 11(10): 2510.
- Zarnowska IM. (2020). Therapeutic Use of the Ketogenic Diet in Refractory Epilepsy: What We Know and What Still Needs to Be Learned. Nutrients. 12, 9: 2616.
- Bielohuby M, Menhofer D, Kirchner H, Stoehr BJ, Muller TD, Stock P, Hempel M, Stemmer K, Pfluger PT, Kienzle E, Christ B, Tschop MH, Bidlingmaier M. (2011). Induction of ketosis in rats fed low-carbohydrate, high-fat diets depends on the relative abundance of dietary fat and protein. Am J Physiol Endocrinol Metab. 300(1): 65–76.
- Ellenbroek JH, van Dijck L, Tons HA, Rabelink TJ, Carlotti F, Ballieux BE, de Koning EJ. (2014). Long-term ketogenic diet causes glucose intolerance and reduced beta- and alpha-cell mass but no weight loss in mice. Am J Physiol Endocrinol Metab. 306(5): E552–E558.
- Jornayvaz FR, Jurczak MJ, Lee H-Y, Birkenfeld AL, FrederickDW, Zhang D, Zhang X-M, Samuel VT, Shulman GI. (2010). A high-fat, ketogenic diet causes hepatic insulin resistance in mice, despite increasing energy expenditure and preventing weight gain. Am J Physiol Endocrinol Metab. 299(5): E808–E815.
- Bielohuby M, Sawitzky M, Stoehr BJ, Stock P, Menhofer D, Ebensing S, Bjerre M, Frystyk J, Binder G, Strasburger C, Wu Z, Christ B, Hoeflich A, Bidlingmaier M. (2011). Lack of dietary carbohydrates induces hepatic growth hormone (GH) resistance in rats. Endocrinology. 152(5): 1948–1960.
- Caton SJ, Bielohuby M, Bai Y, Spangler LJ, Burget L, PflugerP, Reinel C, Czisch M, Reincke M, Obici S, Kienzle E, Tschop MH, Bidlingmaier M. (2012). Low-carbohydrate high-fat diets in combination with daily exercise in rats: effects on body weight regulation, body composition and exercise capacity. Physiol Behav. 106(2): 185–192.
- Xu X, Ding J, Wu X, Huang Z, Kong G, Liu Q, Yang Z, Huang Z, Zhu Q. (2019). Bone microstructure and metabolism changes under the combined intervention of ketogenic diet with intermittent fasting: an in vivo study of rats. Exp Anim. 68(3): 371-380.
- Xiuhua Wu, Zucheng Huang, Xiaomeng Wang, Zhaozong Fu, Junhao Liu, Zhiping Huang, Ganggang Kong, Xiaolin Xu, Jianyang Ding, Qingan Zhu. Ketogenic Diet Compromises Both Cancellous and Cortical Bone Mass in Mice. Calcif Tissue Int.
- Kiliaridis S. (1989). Muscle function as a determinant of mandibular growth in normal and hypocalcaemic rat. Eur J Orthod. 11: 298–308.
- Xu X, Ding J, Wu X, Huang Z, Kong G, Liu Q, Yang Z, Huang Z, Zhu Q. (2019). Bone microstructure and metabolism changes under the combined intervention of ketogenic diet with intermittent fasting: an in vivo study of rats. Exp Anim. 68(3): 371-380.
- Gurnurkar S, Gafen N, Carakushansky M, Vyas N. (2022). LBODP018 Hypercalcemia in A Child on Ketogenic Diet for Intractable Epilepsy. J Endocr Soc.; 6(Suppl 1): A145.
- Stöckly PW, Teuscher U. Orthopaedy combined with activator and skullcap. In: Graber-Swain. Orthodontics. Current principles and techniques. The C.V. Mosby Company. St. Louis-Toronto-Princeton.
- Rabie AB, She TT, Hagg U. (1992). Fal appliance: accelerates and enhances condylar growth. Am J Ortho and Dentofacial Orthop 2003; 123: 40-8.
- A R Ten Cate, A Nancy. (2003). Ten Cate’s Oral Histology. Structure and function. Mosby Inc Editor. Sixth edition. Toronto Princeton.
- Vilman H, Juhl M, Kyrkeby S. (1985). Bone-muscle interactions in the muscular dystrophic mouse. Eu J Orthod. 7: 185-192.
Citation: Garat Juan Abel, Romano Silvia Cristina, Martin Adrián Enrique, Rodriguez Gustavo Martin, Cordoba Maria Lucia, Mir Maria Magali and Salum Maria Karina. (2024). Influence of the Ketogenic Diet on Mandibular Growth and its Potential Impact in Facial Development. A Cephalometric Study in Growing Rats. Journal of Oral Care and Dentistry 5(1).
Copyright: © 2024 Garat Juan Abel. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
