Research Article
Volume 7 Issue 1 - 2025
Localization of Cytokeratin and Smooth muscle actin in the accessory genital glands of camel (Camelus dromedarius) during rutting and non-rutting seasons
1Department of Histology and Cytology, Faculty of Veterinary Medicine, Mansoura University, Egypt
2Department of Anatomy, Faculty of Veterinary Medicine, Mansoura University, Egypt
2Department of Anatomy, Faculty of Veterinary Medicine, Mansoura University, Egypt
*Corresponding Author: Mahmoud Badran Shoaib, Department of Anatomy and Embryology, Faculty of Veterinary Medicine, Mansoura University, Mansoura, Egypt.
Received: December 20, 2024; Published: January 02, 2025
Abstract
The present study has disclosed for the first time the distribution of cytokeratin (CK) and α smooth muscle actin (αSMA) in the accessory genital glands of camel. In prostate, CK was localized in the cytoplasm of columnar cells of secretory acini and in the scanty cytoplasm of basal cells. In the ampulla of ductus deferens, the CK reaction was found in the pseudostratified columnar epithelium of mucosa and in the secretory columnar epithelium of submucosal glands. In the bulbourethral gland, CK reaction was exclusively observed in the pyramidal cells of type A and type C secretory units as well as in the lining epithelium of the duct system. αSMA was localized to the smooth muscle cells of the prostatic capsule, fibromuscular stroma and blood vessels. In the ampulla, αSMA reaction was seen in the smooth muscle of tunica muscularis, fibromuscular stroma and blood vessels. In the bulbourethral gland, αSMA was only localized to the smooth muscle cells of the capsule and blood vessels in both reproductive periods. Unexpectedly, neither the interlobular nor the intralobular connective tissue stroma of bulbourethral gland was reacted to αSMA. In conclusion, the distribution of CK and αSMA in the accessory genital glands of camel might point out to their roles in the male reproduction.
Keywords: Camel; Accessory genital glands; Immunohistochemistry
Introduction
Although the camel plays an important role as a domesticated mammal in the arid regions of Africa, Asia and Australia, many aspects of its reproduction are still unknown (Zayed et al., 1995). In dromedaries, the accessory sex glands are the prostate, the ampulla of ductus deferens, and the bulbourethral glands (Cowper’s), whereas camelids do not have vesicular glands (El Wishy et al., 1972; Ali et al., 1976, 78; Mosallam, 1981; Hafez and Hafez, 2001; El Wakeil, 2010). A detailed morphological description of these glands is given elsewhere (Ali et al., 1976; Mosallam, 1981; El Wakeil, 2010).
Although seasonal changes in the spermatogenic activity are still the subject of dispute (Abdel-Raouf et al., 1975; Tingari et al., 1984), previous investigations generally agree that season of the year have an obvious effect on the morphology of the accessory genital glands in the camel (El Wishy et al., 1972; Ali et al., 1976, 78; Mosallam, 1981; El Wakeil, 2010).
In Egypt, reproductive activity builds up during September and October and the animal is actually in rut during November–February. Activity falls in March, and regresses steadily from April onwards. June, July and August are the months of extreme inactivity (non- rut), but not total quiescence (Abdel-Raouf et al., 1975; Marai et al., 2009; El-Bahrawy and El Hassanein, 2011).
The cytoskeleton of eukaryotic cells is composed of three major protein families that form filamentous structures running throughout the cell, i.e microfilaments (actin isoforms), microtubules made of α- and β-tubulin, and the intermediate filaments (Ramaekers and Bosman, 2004).
Actin, one of the major cytoskeletal components in eukaryotic cells, is a 43-kDa globular monomeric protein that can polymerize into double-helical filaments. Actin occurs in two forms, the globular or G-actin which polymerizes into the other form which is called filamentous or F-actin. In mammals, actin comprises a highly conserved protein that fall into three broad classes:α,β, and γ isoforms. It is mainly located in the cytoplasm, but it is also present in the nucleus (Disanza et al., 2005). Generally, the αSMA is regarded as a marker of terminal smooth muscle differentiation (Skalli et al., 1986). Recent data suggest that αSMA plays a direct role in myofibroblast contractile activity through its N-terminal domain AcEEED (Chaponnier and Gabbiani, 2004). Previously, numerous approaches have studied the localization of αSMA in the prostate of rat (Hayward et al., 1996; Antonioli et al., 2004, 2007) and human (Castellucci et al., 1996; Elbadawi et al., 1997; Jennifer et al., 2002; Tomas and Kruslin ,2004; Taboga et al., 2008).
Cytokeratins (CKs) are the largest subgroup of intermediate filament proteins found in the intracytoplasmic cytoskeleton of epithelial tissue. The CK family is a highly complex multigene family of polypeptides, the molecular weight of which ranges from 40 to 68 kDa. There are two types of CKs: the acidic type I CKs and the basic or neutral type II CKs (Schweizer et al., 2006). CKs act as protein scaffolds with structural and regulatory functions in a cell-type-specific manner, as underscored by keratinopathies (Omary et al., 2004) and knockout mice (Magin et al., 2004; Gu and Coulombe, 2007). Recently, new functions of cytokeratins in cell signaling and intracellular vesicle transport have also been discovered (Bragulla and Homberger, 2009). Several immunohistochemical studies have also detected CKs in the prostate of rat (Hsieh et al., 1992), goat (Weijman et al., 1992), dog (Vos et al., 1992; Lai et al., 2008 a, b) and human (Achtstätfer et al., 1985; Bártek et al., 1986; Kitajima and Tökés, 1986; Wernert et al., 1986, 1987; Srigley et al., 1990; Sherwood et al., 1991; Castellucci et al., 1996).
To our knowledge, no data are available concerning the camel. Therefore, the present study was conducted to the seasonal changes in the camel accessory genital glands during rutting and non-rutting seasons using immunohistochemical technique.
Materials and Methods
The present study was performed on the accessory genital glands of 10 sexually mature and apparently healthy camels. All samples [five samples from animals during rutting months (November–January) and five samples from animals during non-rutting period (June– August)] were collected within 30 min of slaughter in a local (Zagazig) abattoir.
Tissue preparation
Small samples of the accessory genital glands (0.5–1 cm) were fixed in Bouin’s fluid for 24 h. Thereafter, fixed samples were extensively washed in 70% ethanol (3X24 h) to elute fixative before tissue processing to paraffin wax by routine methods. Using a Leitz rotatory microtome, 5 µm-thick sections were cut and mounted on both 3-aminopropyltriethoxysilane- coated and uncoated glass slides. Paraffin wax embedded sections were kept in an incubator at 40 °C until used for conventional hematoxylin and eosin (H&E) staining and immunohistochemical analysis.
Small samples of the accessory genital glands (0.5–1 cm) were fixed in Bouin’s fluid for 24 h. Thereafter, fixed samples were extensively washed in 70% ethanol (3X24 h) to elute fixative before tissue processing to paraffin wax by routine methods. Using a Leitz rotatory microtome, 5 µm-thick sections were cut and mounted on both 3-aminopropyltriethoxysilane- coated and uncoated glass slides. Paraffin wax embedded sections were kept in an incubator at 40 °C until used for conventional hematoxylin and eosin (H&E) staining and immunohistochemical analysis.
Immunohistochemical staining
For the detection of CK and αSMA, a mouse monoclonal primary antibody against CK (Clone MNF116, M0821) and a mouse monoclonal primary antibody against αSMA (Clone 1A4, M0851) (DAKO, Hamburg, Germany) were used. Antigen localization was achieved using the Dako LSAB®+ Kit, peroxidase (LSAB+ Kit, HRP) technique according to the manufacturer instruction. Briefly, 5 µm sections of paraffin-embedded tissues were dewaxed, rehydrated, and rinsed in PBS pH 7.4 (3 x 5 min). For antigen retrieval, the slides were heat treated in microwave at 750 W for two cycles of 7 minutes each in citrate buffer (PH 6.0). Thereafter the sections were allowed to cool at room temperature for 20 minutes. Endogenous peroxidase was blocked by soaking the sections in 3% v/v hydrogen peroxide/distilled water for 10 min at room temperature followed by washing them under running tap water for additional 10 min. Subsequently the slides were equilibrated in PBS pH 7.4 (2 x 5 min). Non- specific antibody binding was minimized by covering the slides with a serum-free protein blocking reagent (DAKO, Hamburg, Germany) for 10 min at room temperature. Sections were then incubated with primary antibody against CK and αSMA diluted 1:100 in antibody diluent (DAKO, Hamburg, Germany) for 1 hr at room temperature. The slides were subsequently rinsed in PBS pH 7.4 (2 x 5 min) followed by incubation with diluted (1:300 in PBS) biotinylated secondary antibody (rabbit anti-mouse IgG) (DAKO, Hamburg, Germany) for 30 min at room temperature. Bound antibodies were visualized using a Dako LSAB®+ Kit, peroxidase (LSAB+ Kit, HRP) technique and diaminobenzidine (DAB) (DAKO, Hamburg, Germany). All incubations were performed in a humidified chamber. Sections were left unstained or counterstained in Mayer’s haematoxylin, dehydrated, and mounted with DPX (Sigma, Munich, Germany). Negative controls were performed by omission of the primary antibody.
For the detection of CK and αSMA, a mouse monoclonal primary antibody against CK (Clone MNF116, M0821) and a mouse monoclonal primary antibody against αSMA (Clone 1A4, M0851) (DAKO, Hamburg, Germany) were used. Antigen localization was achieved using the Dako LSAB®+ Kit, peroxidase (LSAB+ Kit, HRP) technique according to the manufacturer instruction. Briefly, 5 µm sections of paraffin-embedded tissues were dewaxed, rehydrated, and rinsed in PBS pH 7.4 (3 x 5 min). For antigen retrieval, the slides were heat treated in microwave at 750 W for two cycles of 7 minutes each in citrate buffer (PH 6.0). Thereafter the sections were allowed to cool at room temperature for 20 minutes. Endogenous peroxidase was blocked by soaking the sections in 3% v/v hydrogen peroxide/distilled water for 10 min at room temperature followed by washing them under running tap water for additional 10 min. Subsequently the slides were equilibrated in PBS pH 7.4 (2 x 5 min). Non- specific antibody binding was minimized by covering the slides with a serum-free protein blocking reagent (DAKO, Hamburg, Germany) for 10 min at room temperature. Sections were then incubated with primary antibody against CK and αSMA diluted 1:100 in antibody diluent (DAKO, Hamburg, Germany) for 1 hr at room temperature. The slides were subsequently rinsed in PBS pH 7.4 (2 x 5 min) followed by incubation with diluted (1:300 in PBS) biotinylated secondary antibody (rabbit anti-mouse IgG) (DAKO, Hamburg, Germany) for 30 min at room temperature. Bound antibodies were visualized using a Dako LSAB®+ Kit, peroxidase (LSAB+ Kit, HRP) technique and diaminobenzidine (DAB) (DAKO, Hamburg, Germany). All incubations were performed in a humidified chamber. Sections were left unstained or counterstained in Mayer’s haematoxylin, dehydrated, and mounted with DPX (Sigma, Munich, Germany). Negative controls were performed by omission of the primary antibody.
Results (all results were summarized in table 1)
| CK | α-SMA | |||
| Rutting | Non-rutting | Rutting | Non-rutting | |
| Prostate | ||||
|
+++ | ++ | 0 | 0 |
|
+++ | ++ | 0 | 0 |
|
0 | 0 | +++ | ++ |
|
0 | 0 | +++ | ++ |
| Ampulla | ||||
|
+++ | ++ | 0 | 0 |
|
+++ | ++ | 0 | 0 |
|
0 | 0 | +++ | +++ |
|
0 | 0 | +++ | +++ |
|
0 | 0 | +++ | +++ |
| Bulbourethral gland | ||||
|
+ | + | 0 | 0 |
|
+ | + | 0 | 0 |
|
0 | 0 | 0 | 0 |
|
0 | 0 | +++ | +++ |
|
0 | 0 | 0 | 0 |
(0) absence, (+) mild reactivity, (++) moderate reactivity, (+++) strong reactivity
Table 1: Expression of the CK and α-SMA in the accessory genital glands of camel during rutting and no-rutting seasons.
Table 1: Expression of the CK and α-SMA in the accessory genital glands of camel during rutting and no-rutting seasons.
Cytokeratin
In this study the monoclonal mouse anti-human cytokeratin, clone MNF116, is a broad-spectrum anti-keratin reagent reacting with intermediate and low-molecular-weight keratins (directed to CK5, 6, 8, 17, 19), so that cytokeratin isoforms could not be distinguished.
In this study the monoclonal mouse anti-human cytokeratin, clone MNF116, is a broad-spectrum anti-keratin reagent reacting with intermediate and low-molecular-weight keratins (directed to CK5, 6, 8, 17, 19), so that cytokeratin isoforms could not be distinguished.
In rutting season, strong CK positive staining was observed in the cytoplasm of the columnar cells of secretory acini of prostate. In the basal cells, the intense positive staining for CK had a uniform distribution in the scanty cytoplasm. However, CK reaction was absent in the capsule, fibromuscular stroma, and various blood vessels (Figure 1A, B). Similarly, moderate CK staining was observed in the columnar and basal cells of secretory acini in the non-rutting periods. Although, the CK was randomly distributed as a deposit of fine granules throughout the cytoplasm of columnar cells, more intense reaction was seen in the supranuclear region (Figure 1C, D).
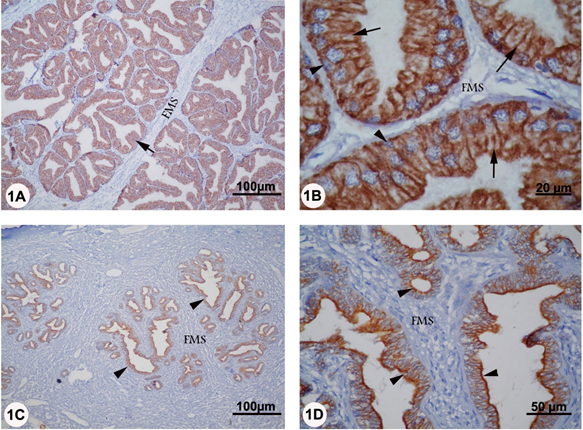
Figure 1A-D: Localization of CK in the prostate of camel during rutting (panel A and B) and non-rutting (panel C and D) periods. Panel A and B: strong CK reactivity was seen in the columnar (arrows) and basal (arrowheads) cells of the prostatic secretory acini. No expression could be detected in fibromuscular septa (FMS). Panel C and D: Moderate CK reactivity was observed in the secretory epithelium of prostate (arrowheads) while no CK staining was evident in the fibromuscular septa (FMS).
Although the ampulla ductus deferens consisted of four tunicae, the CK reaction was only confined to the tunica mucosa and to the ampullary gland of the submucosa in both rutting and non-rutting season (Figure 2 A-D). In rutting period, strong CK staining was localized in the cytoplasm of the pseudostratified columnar epithelium of the tunica mucosa. However, no reaction was seen in the underlying lamina propria. In the submucosa, strong CK staining was seen in the cytoplasm of columnar and basal cells of the secretory units while no staining was evident in the surrounding fibromuscular stroma (Figure 2 A, B). In non-rutting season, moderate CK reaction was also found in the pseudostratified columnar epithelium and in the secretory columnar epithelium of the ampullary gland. However, the CK staining was more intense in the apical cytoplasm of the secretory columnar epithelium of the submucosal ampullary glands (Figure 2 C, D).
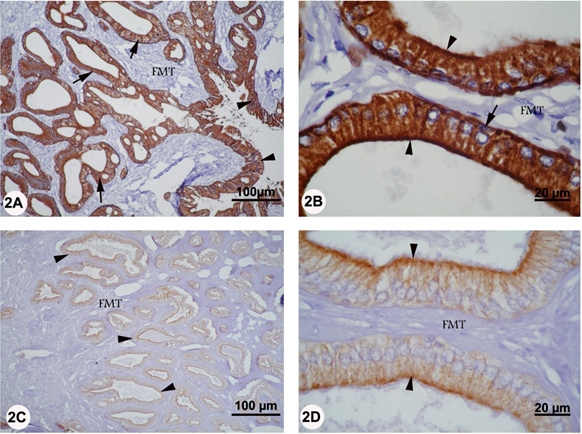
Figure 2A-D: Localization of CK in the ampulla of camel during rutting (panel A and D) and non-rutting (panel C and D) periods. Panel A: The CK reaction was only confined to the tunica mucosa (arrowhead) and to the ampullary gland of the submucosa (arrow). However, no reaction was seen in the fibromuscular tissue (FMT). Panel B: In the submucosa, strong CK reactivity was seen in the cytoplasm of columnar (arrowhead) and basal (arrow) cells of the secretory units while no staining was evident in the fibromuscular tissue (FMT). Panel C: Moderate CK reactivity was found in the secretory columnar epithelium of the ampullary gland (arrowhead). Panel D: The CK staining was more intense in the apical cytoplasm of the secretory columnar epithelium of the submucosal ampullary glands (arrowhead).
In the bulbourethral gland, mild CK reaction was solely observed in the pyramidal cells of type A and type C secretory units as well as in the lining epithelium of the duct system in both rutting and non-rutting periods. No CK staining was however detected in the cuboidal cells of the type B secretory acini and in the surrounding connective tissue stroma (Figure 3 A-D).
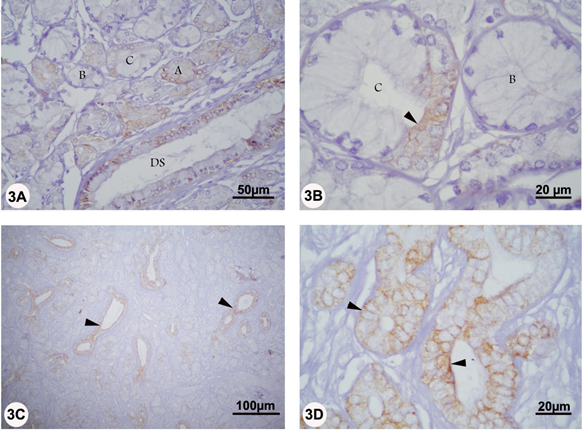
Figure 3A-D: Localization of CK in the bulbourethral gland of camel during rutting (panel A and B) and non-rutting (panel C and D) periods. Panel A and B, mild CK reaction was solely observed in the pyramidal cells of type A and type C secretory units (arrowhead) as well as in the lining epithelium of the duct system (DS). Panel C and D, mild CK reaction was seen in the lining epithelium of the duct system (arrowheads). No CK staining was however detected in the cuboidal cells of the type B secretory acini and in the surrounding connective tissue stroma.
Generally, the glandular tissue and the CK positive staining of all accessory genital glands were comparatively less prominent in the non-rutting period.
α-SMA
In the rutting and non-rutting season, α-SMA was only localized to the smooth muscle cells of the prostatic capsule and fibromuscular stroma immediately surrounding the secretory acini. Additionally, α-SMA staining was seen in the smooth muscle cells of the prostatic blood vessels. α-SMA reaction was apparently more intense in the fibromuscular stroma of rutting prostate (Figure 4 A-D).
In the rutting and non-rutting season, α-SMA was only localized to the smooth muscle cells of the prostatic capsule and fibromuscular stroma immediately surrounding the secretory acini. Additionally, α-SMA staining was seen in the smooth muscle cells of the prostatic blood vessels. α-SMA reaction was apparently more intense in the fibromuscular stroma of rutting prostate (Figure 4 A-D).
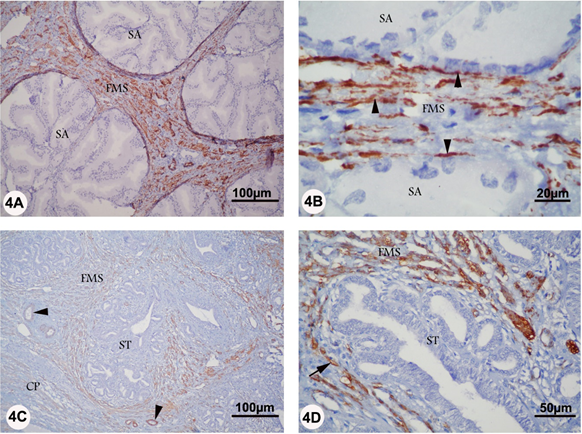
Figure 4A-D: Localization of αSMA in the prostate of camel during rutting (panel A and B) and non-rutting (panel C and D) periods. Panel A and B: αSMA reaction (arrowhead) was localized to the smooth muscle cells of the fibromuscular stroma (FMS) immediately surrounding the secretory acini (SA). Panel C and D, αSMA staining (arrow) was seen in the fibromuscular stroma (FMS) surrounding the secretory tissue (ST), in the smooth muscle cells of prostatic blood vessels (arrowhead), and in the prostatic capsule (CP).
In the ampulla, strong α-SMA reaction was seen in the smooth muscle of tunica muscularis and fibromuscular stroma surrounding the submucosal ampullary gland in both rutting and non-rutting season. Additionally, α-SMA staining was seen in the smooth muscle cells of the ampullary blood vessels (Figure 5 A-D).
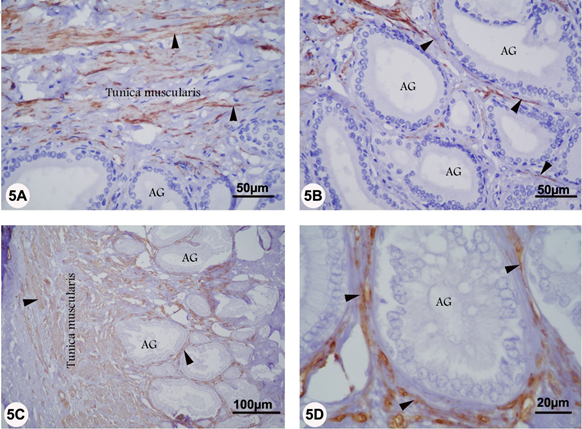
Figure 5 A-D: Localization of αSMA in the ampulla of camel during rutting (panel A and B) and non-rutting (panels C and D) periods. Panels A, B, C and D: Strong ?SMA reaction was seen in the smooth muscle of tunica muscularis and fibromuscular stroma (arrowhead) surrounding the submucosal ampullary gland (AG).
In the Bulbourethral gland, strong α-SMA was only localized to the smooth muscle cells of the capsule and blood vessels in both reproductive periods. Interestingly, neither the interlobular nor the intralobular connective tissue stroma was reacted to α-SMA (Figure 6 A-D).
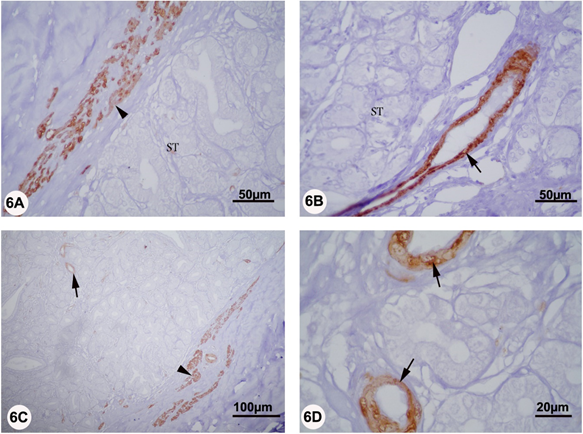
Figure 6 A-D: Localization of αSMA in the bulbourethral of camel during rutting (panel A and B) and non-rutting (panel C and D) periods. Panels A, B, C, and D: Strong αSMA staining was only localized to the smooth muscle cells of the capsule (arrowhead) and blood vessels (arrows)..
Generally, no αSMA staining was evident within the lining epithelium of the secretory units of the accessory genital glands of camel either in rutting or non-rutting period.
Discussion
The cytoskeleton carries out three broad functions: it spatially organizes the contents of the cell; it connects the cell physically and biochemically to the external environment; and it generates coordinated forces that enable the cell to move and change shape (Fletcher and Mullins, 2010). Since the cytoskeleton is involved in virtually all cellular processes, abnormalities in this essential cellular component frequently result in disease (Ramaekers and Bosman, 2004).
Cytokeratins perform instrumental functions within epithelial cells to ensure not only their physical integrity but also their metabolic processes (Vaidya and Kanojia, 2007). The present study represents the first report that concerned the localization of CK and α-SMA in the accessory genital glands of camel. In prostate, CK was generally localized in the cytoplasm of the columnar cells of secretory acini and in the scanty cytoplasm of basal cells in both rutting and non-rutting period. Conversely, no expression was seen in the capsule, fibromuscular stroma, and blood vessels. These findings are consistent with numerous approaches that investigated the localization of CKs in the prostate of several mammalian species including rat (Hsieh et al. 1992), goat (Weijman et al., 1992), dog (Lai et al., 2008 a, b) and human (Achtstätfer et al., 1985; Bártek et al., 1986; Kitajima and Tökés, 1986; Wernert et al., 1986, 1987; Sherwood et al., 1991; Castellucci et al., 1996). In the ampulla of ductus deferens, the CK reaction was found in the pseudostratified columnar epithelium and in the secretory columnar epithelium in both rutting and non-rutting season. However, no reaction was evident in the surrounding connective tissue.
In the bulbourethral gland, CK reaction was exclusively observed in the pyramidal cells of type A and type C secretory units as well as in the lining epithelium of the duct system in both rutting and non-rutting periods. No CK staining was however detected in the cuboidal cells of the type B secretory acini and in the surrounding connective tissue stroma. In human, the available literatures are contradictory. One approach stated that the bulbourethral glands are negative for high-molecular-weight cytokeratin K-903 (34beta E12) (Saboorian et al., 1997). Conversely, other study reported that the high-molecular-weight cytokeratin is strongly reactive with the ductular epithelium and demonstrated an attenuated cell lining at the periphery of lobules (Cina et al., 1997).
Taken together, the localization of CK in the simple epithelium of accessory genital glands of camel in rutting and non-rutting seasons may point out to its crucial role in the male reproduction. In this concept, CK might regulate the secretory functions of such epithelia, act as signaling platforms, and/or protect epithelial cells against mechanical stress. This speculation is substantiated by the findings that the simple epithelia are commonly found lining glands and in organs involved in secretion and absorption, and the individual cells are often polarized, which suggests that the unique expression of simple epithelial cytokeratin in these cells is likely to have functional consequences related to polarized protein sorting, absorption, and secretion (Toivola et al., 2005; Oriolo et al., 2007). Moreover, CKs are involved in cell signaling, cell transport, cell compartmentalization and cell differentiation (Oshima, 2007; Vaidya and Kanojia, 2007). Additionally, the best-known function of CK is to provide a scaffold (through self-bundling and by forming thicker strands) for epithelial cells and tissues to sustain mechanical stress, maintain their structural integrity, ensure mechanical resilience, protect against variations in hydrostatic pressure and establish cell polarity (Coulombe and Omary, 2002; Gu and Coulombe, 2007). CK also influence cell metabolic processes by regulating protein synthesis and cell growth (Gu and Coulombe, 2007) and may be involved in the transport of membrane-bound vesicles in the cytoplasm of epithelial cells (Planko et al., 2007).
In the non-rutting periods, the CK positive staining of all glands specially the prostate was comparatively less prominent as in the rutting period. This variation in CK expression could be attributed to the fluctuated level of androgen hormone during the rutting and non- rutting season. Generally, testosterone levels reach the basal level during the non-rutting season (August), while reach a maximum during the rut (February) (Marai et al., 2009; El- Bahrawy and El Hassanein, 2011). Androgen appears to be a pleiotropic factor in regulating the expression of both luminal and basal specific CK mRNA expression in an organ-specific manner. In rat accessory sex organ, the levels of CK mRNAs expression are negatively regulated by androgen (Hsieh et al., 1992). This discrepancy in the effect of androgen on the accessory genital glands may be species-specific.
Smooth muscle contractile activity is a major regulator of function of the vascular system, respiratory system, gastrointestinal system and the genitourinary systems. Therefore, malfunction of contractility in these systems leads to a host of clinical disorders (Kim et al., 2008). α-SMA is mainly found in cells having contractile functions and is therefore a powerful probe in the study of smooth muscle cell differentiation in normal and pathological conditions (Skalli et al., 1986; van Nassauw et al., 1993).
In the present study, α-SMA was only localized to the smooth muscle cells of the prostatic capsule and fibromuscular stroma immediately surrounding the secretory acini. Additionally, α-SMA staining was seen in the smooth muscle cells of the blood vessels.
Nevertheless, no αSMA was evident within the lining epithelium of the secretory units of the camel prostate either in rutting or non-rutting period. Similar results are also described in the prostate of rat (Hayward et al., 1996; Antonioli et al., 2004, 2007) and human (Castellucci et al., 1996; Elbadawi et al., 1997; Jennifer et al., 2002; Tomas and Kruslin, 2004; Taboga et al., 2008). Our results are also parallel to the previous morphological findings whereas as the interlobular stroma of the camel's prostate is less fibrous and more muscular (Ali et al., 1978; Mosallam, 1981; El Wakeil, 2010).
In the ampulla, α-SMA reaction was seen in the smooth muscle of tunica muscularis and fibromuscular stroma surrounding the submucosal ampullary gland in both rutting and non-rutting season. Additionally, ?-SMA staining was seen in the smooth muscle cells of the ampullary blood vessels. These findings confirm the previous morphological studies on the ampulla whereas the interstitial stroma in the glandular part of the ampulla ductus deferens in camel is formed of reticular fibers in common with smooth muscle fibers (Ali et al., 1978; Mosallam, 1981; El Wakeil, 2010). Similar morphological reports are also described in several mammalians (Cooper and Hamilton, 1977; Riva et al., 1982; Murakami et al., 1986) and non-mammalian (Zalisko and Larsen, 1988) vertebrates. The presence of such great amount of smooth muscle fibers in the interlobular stroma of these glands lead us to think that they are related in some way to the evacuation of the ampullary and prostatic secretion.
In the Bulbourethral gland, α-SMA was only localized to the smooth muscle cells of the capsule and blood vessels in both reproductive periods. Unexpectedly, neither the interlobular nor the intralobular connective tissue stroma was reacted to α-SMA. These results are concurrent with the previous morphological approaches on camel where the compound tubuloalveolar secretory end-pieces of the bulbourethral gland are only supported by abundant reticular fibers (Ali et al., 1978; Mosallam, 1981; El Wakeil, 2010). On the contrary, α-SMA reaction is seen at the periphery of the secretory acini of human bulbourethral gland (Cina et al., 1997; Saboorian et al., 1997). Moreover, myofibroblasts cells are demonstrated in the secretory tissue of bulbourethral glands in rat (Nielsen, 1976), Japanese monkey (Murakami et al., 1981), and human (Hellgren et al., 1982; Riva et al., 1988; Saboorian et al., 1997). Collectively, the evacuation of camel bulbourethral gland may be depending on the capsular musculature and not on the interlobular stroma.
Our study also showed that the basal cells of the secretory acini of prostate and ampullary glands were positively stained with CK while negatively reacted with α-SMA. These findings clearly indicate that the basal cells of these glands are not of myoepithelial origin whereas the secretory acini do not seem to need specialized myoepithelial cells to aid in the expulsion of acinar contents because of the abundant fibromuscular stroma. Similar results are also reported in human (Srigley et al., 1990). Generally, the immunocytochemical characterization of myofibroblasts is based on a combination of different markers, such as expression of α -SMA, vimentin, prolyl 4-hydroxylase and absence of cytokeratin, calponin and desmin immunostaining (Lazard et al., 1993; van der Loop et al., 1996; Wever and Mareel, 2003).
In conclusion, the distribution of CK in the accessory genital glands of camel during rutting and non-rutting seasons might indicate its critical role in the male reproduction. Moreover, the myofibroblasts in the accessory genital glands may provide the major force that allows these glands to evacuate their secretion.
References
- Abdel-Raouf, M., M.R. Fateh El-Bab & M.M. Owaida, (1975). Studies on reproduction in the camel (Camelus dromedarius). V. Morphology of the testis in relation to age and season. Journal of reproduction and fertility, 43: 109–16.
- Achstätfer, T., Moll R, B. Moore & W.W. Frank, (1985). Cytokeratin polypeptide patterns of different epithelia of the human Male urogenital tract: Immunofluorescence and Gel Electrophoretic Studies. The journal of histochemistry and cytochemistry, 33, 415-426.
- Ali, H.A., K.A. Moniem & M.D. Tingari, (1976). Some histochemical studies on the prostate, uretheral and bulbourethral glands of the one-humped camel. The Histochemical journal, 8, 565-578.
- Ali, H.A., M.D.Tingari & K.A.Moniem, (1978). The morphology of the accessory male glands and histochemistry of the ampulla ductus deferentis of the camel (Camelus dromedarius). Journal of anatomy, 125, 277-292
- Antonioli, E., H.H.M. Della-Colleta & H.F. Carvalho, (2004). Smooth Muscle Cell Behavior in the Ventral Prostate of Castrated Rats. Journal of andrology, 25,50–56.
- Antonioli, E., A.B. Cardoso & H.F. Carvalho, (2007). Effects of Long-Term Castration on the Smooth Muscle Cell Phenotype of the Rat Ventral Prostate. Journal of andrology, 28, 777– 783.
- Bártek J., J. Bártková , J. Taylor-Papadimitriou , A .Rejthar , J. Kovarík , Z. Lukás & B. Vojt?sek, (1986). Differential expression of keratin 19 in normal human epithelial tissues revealed by monospecific monoclonal antibodies. The Histochemical journal, 18, 565-75.
- Bragulla, H.H. & D.G. Homberger, (2009). Structure and functions of keratin proteins in simple, stratified, keratinized and cornified epithelia. Journal of anatomy, 214, 516-59.
- Castellucci, E., T. Prayer-Galetti , M. Roelofs , F. Pampinella , L. Faggian ,M.Gardiman , F. Pagano & S.Sartore , (1996). Cytoskeletal and cytocontractile protein composition of stromal tissue in normal, hyperplastic, and neoplastic human prostate. An immunocytochemical study with monoclonal antibodies. Annals of the New York Academy of Sciences, 784, 496-508.
- Chaponnier, C& G. Gabbiani, (2004). Pathological situations characterized by altered actin isoform expression. The Journal of pathology, 204:386-95.
- Cina, S.J., M.A. Silberman , H. Kahane and J.I. Epstein, (1997). Diagnosis of Cowper's glands on prostate needle biopsy. The American journal of surgical pathology, 21, 550-5.
- Cooper, T.G. & D.W. Hamilton, (1977). Phagocytosis of spermatozoa in the terminal region and gland of the vas deferens of the rat. The American journal of anatomy, 150, 247-67.
- Coulombe, P.A. & M.B. Omary , (2002). 'Hard' and 'soft' principles defining the structure, function and regulation of keratin intermediate filaments. Current opinion in cell biology, 14, 110-22.
- Disanza, A., A. Steffen , M. Hertzog , E. Frittoli , K. Rottner & G. Scita, (2005). Actin polymerization machinery: the finish line of signaling networks, the starting point of cellular movement. Cellular and molecular life sciences, 62, 955-70.
- El Wakeil, M.A.M., (2010). Effect of seasonal variations on the histological structure of accessory male genital glands of the Camel (Camelus dromedaries). Ph. D. Thesis, Faculty veterinary medicine, Mansoura Univ, Egypt.
- Elbadawi, A., R. Mathews, J.K. Light & T.M.Wheeler, (1997). Immunohistochemical and ultrastructural study of Rhabdosphincter component of the prostatic capsule. The Journal of urology, 158, 1819-1828.
- El-Bahrawy, K.A. & E.E. El Hassanein, (2011). Seasonal Variations of Some Blood and Seminal Plasma Biochemical Parameters of Male Dromedary Camels. American-Eurasian journal of agricultural and environmental sciences, 10, 354-360.
- El-Wishy, A.B., A.M. Mobarak & S.M. Fouad, (1972). The accessory genital organs of the one humped male camel (Camelus dromedarius). Anatomischer Anzeiger, 131, 1-12.
- Fletcher D.A, & R.D.Mullins, (2010). Cell mechanics and the cytoskeleton. Nature, 463, 485- 92.
- Gu, L.H & P.A. Coulombe, (2007). Keratin function in skin epithelia: a broadening palette with surprising shades. Current opinion in cell biology, 19, 13-23.
- Hafez, E.S. & B. Hafez, (2001). Reproductive parameters of male dromedary and bactrian camels. Archives of andrology, 46, 85-98.
- Hayward, S.W, L.S. Baskin , P.C. Haughney , B.A. Foster , A.R. Cunha , G.S. Dahiya R, Prins & G.R. Cunha , (1996). Stromal development in the ventral prostate, anterior prostate and seminal vesicle of the rat. Acta anatomica (Basel), 155, 94-103.
- Hellgren, L., E. Mylius & J.Vincent, (1982). The ultrastructure of the human bulbourethral gland. Journal of submicroscopic cytology, 14, 683-689.
- Hsieh, J.T., H.E. Zhau , X.H.Wang, C.C. Liew & L.W. Chung, (1992). Regulation of basal and luminal cell-specific cytokeratin expression in rat accessory sex organs. Evidence for a new class of androgen-repressed genes and insight into their pairwise control. The Journal of biological chemistry, 267, 2303-10.
- Jennifer, A.T., E.A.Gustavo, J.S. Megan, Vincent C. Smith, Truong D. Dang & David R. Rowley, (2002). Reactive stroma in Human Prostate Cancer: Induction of myofibroblast Phenotype and Extracellular Matrix Remodeling. Clinical cancer research, 8, 2912–2923.
- Kim, H.R., S. Appel, S. Vetterkind & K. G. Morgan, (2008). Smooth muscle signaling pathways in health and disease. Journal of cellular and molecular medicine, 12, 2165–2180.
- Kitajima, K. & Z.A.Tökés, (1986). Immunohistochemical localization of keratin in human prostate. Prostate, 9, 183-90.
- Lai, C.L, van den Ham R, G. van Leenders , der Lugt J van, J.A. Mol & E .Teske, (2008a). Comparative Characterization of the Canine Normal Prostate in Intact and Castrated Animals. The Prostate, 68, 498 -507.
- Lai, C.L., R.van den Ham, G. van Leenders, der Lugt J van, & E.Teske, (2008b). Histopathological and Immunohistochemical Characterization of Canine Prostate Cancer. The Prostate, 68, 477-488.
- Lazard, D., X. Sastre, M.G. Frid, M.A. Glukhova, J.P. Thiery & V.E. Koteliansky, (1993). Expression of smooth muscle-specific proteins in myoepithelium and stromal myofibroblasts of normal and malignant human breast tissue. Proceedings of the National Academy of Sciences of the United States of America, 90, 999–1003.
- Magin, T.M., M. Hesse , R. Meier-Bornheim & J. Reichelt, (2004). Developing mouse models to study intermediate filament function. Methods in cell biology, 78, 65-94.
- Marai, I.F.M., A.E.B. Zeidan, A.M. Abdel-Samee, A. Abizaid & A. Fadiel, (2009). Camels’ reproductive and physiological performance traits as affected by environmental conditions. Tropical and Subtropical Agroecosystems, 10, 129–149.
- Mosallam, A., (1981). Histological and histochemical studies on accessory male genital gland of one humped camel (Camelus dromedrius) Ph. D. Thesis, Cairo University, Egypt.
- Murakami, M., T. Nishida, M. Shiromoto & T.Inokuchi , (1986). Scanning and transmission electron microscopic study of the ampullary region of the dog vas deferens, with special reference to epithelial phagocytosis of spermatozoa and latex beads. Anatomischer Anzeiger, 162, 289-96.
- Murakami, M., A. Sugito, j. Abe, M.hamasaki & T .Shimoka, (1981). SEM observations of some exocrine glands, with special reference to configuration of the associated myoepithelial cells. Biomedical research, 2(Suppl.), 96-102.
- Nielsen, E.H., (1976). The bulbourethral gland of the rat. Fine structure and histochemistry. Anatomischer Anzeiger, 139, 254-63.
- Omary, M.B., P.A. Coulombe , & W.H. McLean , (2004). Intermediate filament proteins and their associated diseases. The New England journal of medicine, 351, 2087-100.
- Oriolo, A.S., F.A.Wald, V.P. Ramsauer & P.J.Salas, (2007). Intermediate filaments: a role in epithelial polarity. Experimental cell research, 313, 2255-64.
- Oshima, R.G., (2007). Intermediate filaments: a historical perspective. Experimental cell research, 313, 1981-94.
- Planko, L., K.Böhse , J. Höhfeld , R.C. Betz , S. Hanneken , S .Eigelshoven , R. Kruse , M.M. Nöthen & T.M.Magin, (2007). Identification of a keratin-associated protein with a putative role in vesicle transport. European journal of cell biology, 86, 827-39.
- Ramaekers, F.C. & F.T.Bosman, (2004). The cytoskeleton and disease. J Pathol 204, 351-4 Riva, A., F. Testa-Riva , E. Usai and M. Cossu , 1982. The ampulla ductus deferentis in man, as viewed by SEM and TEM. Archives of andrology, 8, 157-64.
- Riva, A., E. Usai , M. Cossu , R. Scarpa & F. Testa-Riva, (1988). The human bulbo-urethral glands. A transmission electron microscopy and scanning electron microscopy study. Journal of andrology, 9, 133-41.
- Saboorian, M.H., H. Huffman , R. Ashfaq , A.G. Ayala & J.Y. Ro, (1997). Distinguishing Cowper's glands from neoplastic and pseudoneoplastic lesions of prostate: immunohistochemical and ultrastructural studies. The American journal of surgical pathology, 21, 1069-74.
- Schweizer, J., P.E. Bowden , P.A.Coulombe , L. Langbein , E.B. Lane , T.M. Magin , L .Maltais , M.B. Omary , D.A. Parry , M.A. Rogers & M.W.Wright, (2006). New consensus nomenclature for mammalian keratins. The Journal of cell biology, 174, 169-74.
- Sherwood, E.R., G.Theyer , G. Steiner , L.A .Berg , J.M. Kozlowski & C.Lee, (1991). Differential expression of specific cytokeratin polypeptides in the basal and luminal epithelia of the human prostate. Prostate, 18, 303-14.
- Skalli, O., P. Ropraz , A. Trzeciak , G. Benzonana , D .Gillessen & G. A.Gabbiani, (1986). A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. The Journal of cell biology, 103, 2787-96.
- Srigley, J.R., I. Dardick , R.W. Hartwick & L. Klotz, (1990). Basal epithelial cells of human prostate gland are not myoepithelial cells. A comparative immunohistochemical and ultrastructural study with the human salivary gland. The American journal of pathology, 136, 957-66.
- Taboga, S.R., E. Scortegagna, Mp. Siviero& H.F.Carvalho, (2008). Anatomy of Smooth Muscle Cells in Nonmalignant and Malignant Human Prostate Tissue. The Anatomical record, 291, 1115–1123.
- Tingari, M.D., A.S. Ramos, E.S.E. Gaili, B.A. Rahma & A.H. Saad, (1984). Morphology of the testis of the one humped camel in relation to reproductive activity. Journal of anatomy, 139, 133–43.
- Toivola, D.M., G.Z. Tao , J. Habtezion ALiao & M.B. Omary , (2005). Cellular integrity plus: organelle-related and protein-targeting functions of intermediate filaments. Trends in cell biology, 15, 608-17.
- Tomas, D. & B.Krušlin, (2004). The Potential value of (Myo) Fibroblastic stromal reaction in the diagnosis of prostatic Adenocarcinoma. The Prostate, 61, 324 -331.
- Vaidya M.M. , & D. Kanojia , (2007). Keratins: markers of cell differentiation or regulators of cell differentiation? Journal of biosciences, 32, 629-34.
- van der Loop F.T.L., G. Schaart, E.D.J. Timmer, F.C.S. Ramaekers & van Eys GJJM. Smoothelin, (1996). Smoothelin: A novel cytoskeletal protein specific for smooth muscle cells.The Journal of cell biology, 134, 401–411.
- van Nassauw L., F. Harrisson & M. Callebaut, (1993). Smooth muscle cells in the peritubular tissue of the quail testis. European journal of morphology, 31, 60-4.
- Vos J.H., T.S. van den Ingh , M. de Neijs , F.N. van Mil , D. Ivanyi & F.C. Ramaekers , (1992). Immunohistochemistry with keratin monoclonal antibodies in canine tissues: urogenital tract, respiratory tract, (neuro-) endocrine tissues, choroid plexus and spinal cord. Zentralblatt für Veterinärmedizin. Reihe A, 39, 721-40.
- Weijman, J., F.C. Ramaekers , T.A. Elsinghorst , P.J. van Wichen & P. Zwart, (1992). Changing cytokeratin expression patterns in diethylstilbestrol dipropionate-induced metaplastic lesions of the goat prostate. The Veterinary quarterly, 14, 2-7.
- Wernert, N., G. Seitz & T. Achtstätter, (1987). Immunohistochemical investigation of different cytokeratins ,and vimentin in the prostate from the fetal period up to adulthood and in prostate carcinoma. Pathology, research and practice, 182, 617-26.
- Wernert, N., G. Seitz, R. Goebbels & G. Dhom, (1986). Immunohistochemical demonstration of cytokeratins in the human prostate. Pathology, research and practice, 181, 668-74.
- Wever, D.O. & M.Mareel, (2003). Role of tissue stroma in cancer cell invasion. The Journal of pathology, 200, 429–447.
- Zalisko, E.J. & J.H. Larsen, (1988). Ultrastructure and histochemistry of the vas deferens of the salamander Rhyacotriton olympicus: adaptations for sperm storage. Scanning microscopy, 2, 1089-95.
- Zayed, A.E., A. Hifny, A. Abou-Elmagd & K.H.Wrobel, (1995). Seasonal changes in the intertubular tissue of the camel testis (Camelus dromedarius). Annals of anatomy, 177, 112– 99.
Citation: Abd-Elmaksoud Ahmed, S. Ebada Mohamed and Mahmoud Badran Shoaib. (2025). “Localization of Cytokeratin and Smooth muscle actin in the accessory genital glands of camel (Camelus dromedarius) during rutting and non-rutting seasons”. Journal of Medicine and Surgical Sciences 7.1.
Copyright: © 2025 Mahmoud Badran Shoaib. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
