Editorial
Volume 6 Issue 1 - 2024
Blubbery and Suety-Steroid Cell Tumour Ovary
A.B. Diagnostics A-1, Rajouri Garden, New Delhi 110027 India
*Corresponding Author: Anubha Bajaj, A.B. Diagnostics A-1, Rajouri Garden, New Delhi 110027 India.
Received: December 05, 2024; Published: December 12, 2024
Ovarian steroid cell tumour emerges as an exceptionally encountered, preponderantly unilateral, stromal neoplasm constituted of steroid cells. Additionally, designated as steroid cell tumour, not otherwise specified (NOS), neoplasm is associated with a potential for malignant metamorphosis. An estimated 50% lesions delineate androgenic manifestations. Tumefaction is constituted of diffuse sheets of polygonal to spherical tumour cells imbued with moderate to abundant cytoplasm. Constituting < 1% of ovarian tumours, average age of disease emergence is 43 years whereas tumours associated with von Hippel-Lindau disease may occur at 27 years. An estimated ~43% instances appear clinically malignant wherein malignant neoplasms are discerned in elderly subjects. Initial two decades of life appear devoid of occurrence of malignant ovarian steroid cell tumour.
Steroid cell tumour commonly arises within the ovary wherein extra-ovarian genesis is extremely exceptional (1,2). Neoplasm is posited to arise from ovarian stromal cells. Tumefaction arises due to dysregulation within hypoxia signalling pathway. Aberrant VHL gene ensues within instances concordant von Hippel-Lindau disease (1,2). Tumefaction depicts pathogenic variants pertaining to chromosomal mutations within diverse hypoxia related genes as VHL, SRC, IDH2, HIF1A, SDHB and FOXO4. Besides, genetic mutations within FH, CTTNB1, CASP10 or P53 genes may be enunciated (1,2). Malignant steroid cell tumour demonstrates genomic instability, co-amplification of MDM1/CDK4, genomic rearrangements within ATRX, copy number gains within MDM2 / CDK4 or chromosomal mutation within BAP1(1,2).
An estimated ~50% neoplasms appear androgenic. Oestrogenic manifestations are exceptional and associated with isosexual pseudo-precocity, occasional progestogen-like alterations, Cushing’s syndrome or elevated serum cortisol levels. Infrequently, subjects depict hypercalcemia, erythrocytosis, ascites or acute heart failure. Ovarian steroid cell tumour is concordant with type I and type II von Hippel-Lindau disease wherein symptoms as abnormal uterine bleeding, amenorrhea and infertility are commonly encountered (1,2).
Cytological examination exhibits sheets of enlarged, spherical to polygonal cells adherent to vascular fragments of stromal tissue. Tumour cells are incorporated with abundant, granular to pale foamy cytoplasm and miniature, spherical, centric nuclei with conspicuous nucleoli. Malignant steroid cell tumour with ascites delineates clusters of or isolated tumour cells wherein malignant cells display minimal overlapping and cannibalism (2,3). Frozen section exemplifies diffuse cellular dissemination or a distinct, nested arrangement of tumour cells. Tumour cells are impregnated with abundant, eosinophilic or clear, foamy cytoplasm (2,3). Grossly, neoplasm is predominantly unilateral, solid, well circumscribed and infrequently lobulated. Tumour magnitude is variable with mean tumour magnitude of 8.4 centimetres. Cut section exhibits foci of lipid rich(yellow/orange), lipid poor(red/brown) or abundant lipochrome pigment (dark brown/black) areas.
Occasionally, focal necrosis, haemorrhage and cystic degeneration may ensue (2,3). Upon microscopy, neoplasm commonly depicts a diffuse architecture. Infrequently, nests, cords, pseudo-glandular structures or follicle-like tumour configuration is observed. Generally, stroma is sparse. Besides, fibrotic, hyalinized, oedematous or myxoid stroma may be discerned. Exceptionally, stromal calcification or psammoma bodies may be exemplified (2,3). Tumour cells appear polygonal to spherical with moderate to abundant cytoplasm, distinct cellular perimeter and centric nuclei. Lipid poor tumour cells depict eosinophilic and granular cytoplasm whereas lipid rich cells display vacuolated and spongy cytoplasm. Neoplasms demonstrating an admixture of aforesaid cell types may be discerned. In contrast to diverse subtypes of steroid cell tumour, ovarian subtype is preponderantly composed of cells with lipid rich cytoplasm. Nearly 40% neoplasms demonstrate lipochrome pigment. Signet ring cells are exceptionally encountered. Adipocytic metaplasia or hyaline globules are infrequent (2,3).
Commonly, tumour cells depict minimal to absent nuclear atypia along with minimal mitotic activity < 2 mitotic figures/10 high power fields. Grade I to grade III tumours demonstrate nuclear atypia along with enhanced mitotic activity with ~15 mitotic figures/10 high power fields. Focal necrosis and haemorrhage are occasional and are especially encountered within tumours demonstrating significant cytological atypia (3,4). Tumour metastasis pre-eminently recapitulates the primary tumour although poorly differentiated metastasis may be discerned. Miniature tumours may delineate stromal hyperthecosis within adjacent ovarian stroma and contralateral ovary (3,4).
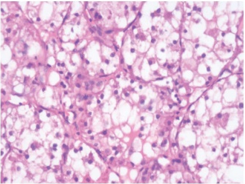
Figure 1: Steroid cell tumour with malignant features depicting nests and cords of lipid rich cells with vacuolated cytoplasm and eccentric, compressed nuclei traversed by fibrous tissue septa (6).
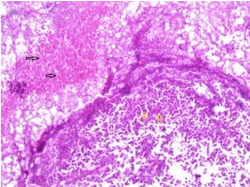
Figure 1: Steroid cell tumour ovary delineating cords and nests of lipid rich cells imbued with vacuolated cells and eccentric, compressed nuclei segregated by mature fibrous tissue septa (7).
| Stage I | T1 | N0 | M0 | |
| Stage IA | T1a | N0 | M0 | |
| Stage IB | T1b | N0 | M0 | |
| Stage IC | T1c | N0 | M0 | |
| Stage II | T2 | N0 | M0 | |
| Stage IIA | T2a | N0 | M0 | |
| Stage IIB | T2b | N0 | M0 | |
| Stage IIIA1 | T1/2 | N1 | M0 | |
| Stage IIIA2 | T3a | Any N | M0 | |
| Stage IIIB | T3b | Any N | M0 | |
| Stage IIIC | T3c | Any N | M0 | |
| Stage IV | Any T | Any N | M1 | |
| Stage IVA | Any T | Any N | M1a | |
| Stage IVB | Any T | Any N | M1b |
Table 1: Staging of Ovarian tumours as per American Joint Committee on Cancer (AJCC) Eighth edition (3,4).
Ovarian steroid cell tumour appears immune reactive to inhibin, calretinin, steroidogenic factor 1(SF1), Melan A, androgen receptor, vimentin or CD99. Tumour cells appear immune non-reactive to FOXL2, PAX8, synaptophysin, chromogranin, carcinoembryonic antigen (CEA) and epithelial membrane antigen (EMA) (4,5).
Ovarian steroid cell tumour requires segregation from neoplasms as ovarian clear cell carcinoma, metastatic renal cell carcinoma, pregnancy luteoma, Leydig cell tumour, Sertoli cell tumour, metastatic malignant melanoma, oxyphilic struma ovarii or hepatoid yolk sac tumour. Neoplasm may appropriately be discerned upon cogent histological assessment (4,5). Subjects demonstrating androgenic manifestations along with or devoid of Cushing’s syndrome expound elevated urinary levels of 17-ketosteroids and 17-hydroxycorticosteroids, serum testosterone and androstenedione. Cushing’s syndrome depicts elevated serum or urinary free cortisol (4,5). Upon ultrasonography, a hypoechoic or isoechoic tumefaction with homogenous or heterogeneous texture is exemplified. Characteristically, neoplasm expounds abundant blood flow signals. Computerized tomography (CT) expresses areas of minimal density pertaining to lipid content of the tumefaction (4,5). Upon T2 weighted magnetic resonance imaging (MRI), tumefaction delineates intermediate signal intensity and avid contrast enhancement, a feature which is indicative of hyper-vascularity of the neoplasm. Out of phase MR imaging demonstrates signal loss, contingent to lipid content of the tumefaction (4,5). Generally, features discerned upon computerized tomography (CT) and magnetic resonance imaging (MRI) appear variable and are contingent to quantifiable lipid component of the tumour vis-a vis fibrous stroma. Ovarian steroid cell tumour may appropriately be subjected to surgical manoeuvers as unilateral salpingo-oophorectomy, especially in subjects where fertility sparing is necessitated. Alternatively, total hysterectomy and bilateral salpingo-oophorectomy may be beneficially employed. Adjuvant chemotherapy may be adopted for alleviating malignant neoplasms (4,5). Neoplasms associated with reoccurrence or disease progression may be suitably managed with combination of surgical excision, chemotherapy and gonadotropin releasing hormone (GnRH) analogue therapy (4,5). Tumour reoccurrence may exceptionally be encountered. Upon surgical intervention, few neoplasms may display extra-ovarian dissemination. Tumours with Cushing’s disease may demonstrate extensive intra-abdominal tumour dissemination (4,5). Morphological features concurrent with malignant metamorphosis are expounded as ~two or mitotic figures per 10 high power fields ~tumour necrosis ~tumour magnitude ≥7 centimetres ~intra-tumoral haemorrhage ~grade II or grade III nuclear atypia ~aggressive biological behaviour with ≥ four aforesaid malignant features (4,5).
References
- Wei CH, Fadare O. (2024). Ovarian steroid cell tumors: what do we know so far? Front Oncol. ;14: 1331903.
- Fadare O, Fard EV, Bhargava R et al. (2024). The Malignant Potential of Ovarian Steroid Cell Tumors Revisited: A Multi-institutional Clinicopathologic Analysis of 115 Cases. Am J Surg Pathol. 48(5): 570-580.
- Kong M, Xu X, Xiang L et al. (2024). Case report: Ovarian steroid cell tumor with CA72-4 elevated. Gynecol Endocrinol. 40(1): 2400943.
- Mendoza RP, Wang P, Smith HL et al. (2023). Clinicopathologic Analysis and Molecular Profiling of Ovarian Steroid Cell Tumors. Am J Surg Pathol. 47(12): 1398-1408.
- Plett H, Ricciardi E, Vacaru V et al. (2023). Adult ovarian granulosa cell tumors: analysis of outcomes and risk factors for recurrence. Int J Gynecol Cancer. 33(5):734-740. 6) Image 1 Courtesy: Spandidos publications 7) Image 2 Courtesy: BMC Women’s Health Biomed Central
Citation: Anubha Bajaj. (2024). “Blubbery and Suety-Steroid Cell Tumour Ovary”. Journal of Medicine and Surgical Sciences 6.1.
Copyright: © 2024 Anubha Bajaj. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
