Case Report
Volume 6 Issue 1 - 2024
Recurrent/Disseminated Choroid Plexus Carcinoma: Overall Survival of > 23.6 Years in a One-Year and Nine-Month-Old Female Treated with Antineoplastons.
1Medical Division, Burzynski Clinic, Houston, Texas, USA
2Oncology Writings, Calera, Alabama, USA
2Oncology Writings, Calera, Alabama, USA
*Corresponding Author: Stanislaw R. Burzynski, MD, PhD, Director, Burzynski Clinic; 9432 Katy Freeway, Houston, Texas, USA.
Received: April 29, 2024; Published: May 09, 2024
Abstract
Choroid plexus neoplasms (CPNs) are uncommon and have three types: papilloma, atypical papilloma, and carcinoma (CPC). There is no established treatment for recurrent/disseminated CPC. Objectives: CPN patients received treatment at the Burzynski Clinic (BC) according to the phase II protocol, BT-26, which aimed to evaluate the antitumor activity of ANP and to evaluate patient tolerance to ANP. Tumor response was assessed by sequential brain and spine MRIs utilizing gadolinium enhancement. Findings: After gross total resection of her CPC at another facility, this one-year and nine-month-old child, along with her parents, came to the BC for evaluation and treatment of the child’s recurrent/disseminated CPC. Baseline MRI of the brain (September 15, 2000) showed four enhancing lesions totaling 2.16 cm2 in size. Baseline MRI of the spine (August 28, 2000) showed enhancing but non-measurable dural lesions. Subsequent MRIs (December 14, 2000) showed enhancing brain tumors totaling 3.03 cm2 in size (a 40.3% increase from baseline) and enhancing dural lesions of the spine totaling 0.81 cm2 in size, indicating progressive disease. However, following ANP, this child has enjoyed > 23.6 years overall survival while experiencing good health. Conclusions: A very rare long-term survival with recurrent/disseminated CPC is presented and the usefulness of ANP is discussed.
Keywords: Antineoplastons; Brain tumor; Choroid plexus neoplasm; Phase II study; Recurrent/disseminated choroid plexus carcinoma
Abbreviations: ANP: Antineoplaston therapy; Antineoplastons: Antineoplastons A10 and AS2-1; A10: Atengenal; AS2-1: Astugenal; BC: Burzynski Clinic; CPN: Choroid plexus neoplasm; CPC: Choroid plexus carcinoma; CR: Complete response; CSF: Cerebrospinal fluid; PD: Progressive disease; FDA: Food and Drug Administration; GBM: Glioblastoma; GTR: Gross total resection; ICH: International Conference on Harmonization; IND: Investigational new drug; LPS: Lansky Performance Score; MRI: Magnetic resonance imaging; OR: Objective response; OS: Overall survival; PR: Partial response; RT: Radiation therapy; SAE: Serious adverse event; WHO: World Health Organization
Introduction
Choroid plexus neoplasms (CPNs) are rare but account for 12–20% of brain tumors in children less than one year of age. [1,2]. The choroid plexus consists of highly vascularized tissue, which covers the walls of the ventricles. [3,4] This tissue produces cerebrospinal fluid (CSF) and contains stem cells. [3] The World Health Organization (WHO) classifies a CPN as either a choroid plexus papilloma, an atypical choroid plexus papilloma, or a choroid plexus carcinoma (CPC). [5]
CPCs, the focus of this paper, show elevated mitotic activity, dense cellularity, and brain invasion [6]. Craniospinal dissemination occurs in 12–30% of cases. [6] CPCs have five-year survival rates of 71%, but surviving patients frequently demonstrate developmental and cognitive disabilities. Survival data for recurrent/disseminated CPC is not available. [6] Gross total resection (GTR) of the tumor is the current treatment of choice for CPC. [7] Treatment of hydrocephalus is required in most cases. [7] Radiation therapy (RT) and chemotherapy are also utilized depending on the clinical circumstances. However, further data is needed concerning these treatment modalities. [8] Serial magnetic resonance imaging (MRI) of the brain are used in follow-up after primary therapy. [9]
CPNs are best imaged by MRI scans of the brain with gadolinium. Forty percent of CPNs are in the lateral ventricles. [9] They are hypo- or isointense on T1 weighted images, hyper- or isointense on T2 weighted images, and moderately or strongly enhanced on post-contrast images. [9] Hydrocephalus, seen in approximately 60% of cases, is primarily a result of obstruction, but is also due to increased CSF production and decreased reabsorption. [9]
Ventricular CPNs present with symptoms specifically due to their producing hydrocephalus with increased intracranial pressure. These symptoms include a bulging frontal fontanelle, increased cranial size, headache, nausea and vomiting, and papilledema. [7]
We present here the use of ANP (IV Antineoplaston therapy), which consists of Antineoplaston A10 (Atengenal) and Antineoplaston AS2-1 (Astugenal), in the treatment of a recurrent/disseminated CPC in a one-year and nine-month-old child following GTR.
Materials and Methods
A one-year and 9-month-old female child was in good health until July 2000, when she developed intermittent vomiting and increased head circumference. She underwent an MRI of the brain elsewhere on August 13, 2000, which showed a left-sided intraventricular tumor with hydrocephalus. That same day, the child underwent GTR and placement of an external ventriculostomy. An Ommaya reservoir was subsequently placed. Examination of the microscopic sections of the surgical specimen revealed a CPC. Follow-up MRI of the brain on August 31, 2000, showed progressive/disseminated disease and the child’s parents determined that their daughter be treated at the Burzynski Clinic (BC). She was evaluated there on September 21, 2000, having had only surgical treatment up to that time. The child’s mother described her daughter as having headaches, weakness, and balance problems. The physical examination was essentially normal except for a surgical scar and an Ommaya reservoir in the left parietal region.
The patient had a low LPS, and following baseline MRIs (see “Results”), started treatment on September 22, 2000, as a Special Exception, according to Protocol BT-26, “Phase II Study of Antineoplastons A10 and AS2-1 Infusions in Patients with Choroid Plexus Neoplasms”. In this single arm study, ANP was delivered every four hours via a subclavian catheter and a programmable infusion pump.
The objectives of BT-26 were to 1) “demonstrate the antitumor activity of Antineoplastons A10 and AS2-1 in the treatment of patients with choroid plexus neoplasms by determining the proportion of patients who experience an objective tumor response” and 2) “evaluate the adverse effects and tolerance of Antineoplastons A-10 and AS2-1 in these patients”. Eligibility criteria for BT-26 included 1) Histologic diagnosis of a CPN; 2) Tumor size ≥ 5mm; 3) Age ≥ 6 months; 4) Lansky Performance Status (LPS) of 60% to 100%; and 5) Life expectancy ≥ 2 months.
Gadolinium-enhanced MRI of the brain and spine were used in the diagnosis and follow-up of the patient’s CPC. They were performed every 8 weeks for the first two years and then less frequently. T2-weighted, T2-fluid attenuated inversion recovery (T2-FLAIR), T1 weighted, and T1-weighted contrast-enhanced images were obtained. CPCs exhibit patchy gadolinium-enhancing and sequential T1-weighted contrast-enhanced images were utilized to determine the effect of therapy. [10]
As determined by MRI of the brain, the product of the two greatest perpendicular diameters of each measurable (≥ 5mm) and enhancing lesion was calculated. Tumor size was defined as the sum of these products [11,12]. The response criteria were as follows: a complete response (CR) indicated complete disappearance of all enhancing tumor while a partial response (PR) indicated a 50% or greater reduction in total measurable and enhancing tumor size. CR and PR required a confirmatory brain MRI performed at least four weeks after the initial finding. Progressive disease (PD) indicated a 25% or greater increase in total measurable and enhancing tumor size, or new measurable and enhancing disease, while stable disease (SD) did not meet the criteria for PR or PD [11].
This Phase II trial was conducted in accordance with the U.S. Code of Federal Regulations, Title 21, Parts 11, 50, 56 and 312; the Declaration of Helsinki (1964) including all amendments and revisions; the Good Clinical Practices: Consolidated Guideline (E6), International Conference on Harmonization (ICH) and Guidance for Industry (FDA). By participating in this study protocol, the investigators agreed to provide access to all appropriate documents for monitoring, auditing, IRB review and review by any authorized regulatory agency.
Results
Baseline MRI of the brain performed September 15, 2000, and spine, performed August 28, 2000, (Figure 1) showed four measurable and enhancing brain lesions (right temporal, left temporal, left sylvian fissure, and left occipital) totaling 2.16 cm2 in size and enhancing, but non-measurable dural lesions at the level of the third and fourth thoracic vertebrae and the first and fifth lumbar vertebrae. As described above, this one-year and nine-month-old child was accrued to BT-26, as a Special Exception, and began treatment on September 22, 2000. The starting dose of A10 was 0.79 g/kg/d. It was gradually increased to 23.00 g/kg/d and subsequently reduced to 18.14 g/kg/d. The starting dose of AS2-1 was 0.21 g/kg/d. It was gradually increased to 0.65 g/kg/d and subsequently reduced to 0.56 g/kg/d.
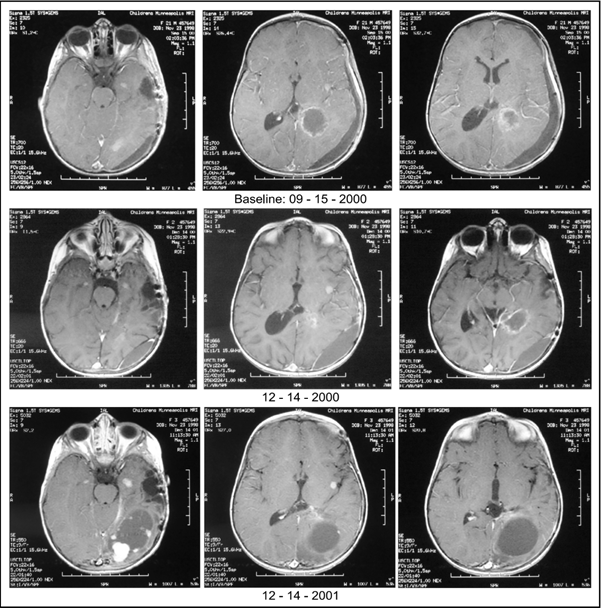
Figure 1: Baseline MRIs: Baseline MRI of the brain performed September 15, 2000 showed four enhancing brain lesions totaling 2.16 cm2 in size while baseline MRI of the spine performed August 28, 2000 (not shown) detailed enhancing, but non-measurable, dural lesions; December 14, 2000: MRIs showed enhancing brain tumors totaling 3.03 cm2 in size (a 40.3% increase from baseline) and enhancing dural lesions of the spine totaling 0.81 cm2 in size (not shown), indicating PD; December 14, 2001: MRIs showed enhancing brain tumors totaling 23.80 cm2 (a 1001.9% increase from baseline) and enhancing dural lesions of the spine totaling 1.12 cm2 in size indicating further progression of disease. MRI = Magnetic resonance imaging; PD = Progressive disease.
On December 14, 2000, MRIs showed enhancing brain tumors totaling 3.03 cm2 in size (a 40.3% increase from baseline) and enhancing dural lesions of the spine totaling 0.81 cm2 in size, indicating PD. ANP was discontinued on July 3, 2001. Final MRIs performed on December 14, 2001, showed enhancing brain tumors totaling 23.80 cm (a 1001.9% increase from baseline) and enhancing dural lesions of the spine totaling 1.12 cm2 in size indicating further progression of disease. The patient has subsequently received no anti-cancer therapy. The date of last contact was April 17, 2024, the patient was doing well and enjoying life. Her overall survival (OS) at that time was 26.6 years.
Adverse events were graded according to the Common Terminology Criteria for Adverse Events Version 3.0. The patient presented here experienced six serious adverse events (SAEs), of which two were thought to be related to ANP (diarrhea, hypokalemia). She fully recovered from all SAEs.
Consent was obtained from the patient for publication of the brain/spine MRI images (Figure 1) and the post-therapy photograph (Figure 2) presented in this report.
Discussion
Chemotherapy, RT, and a second surgical procedure have been utilized in the treatment of recurrent CPC. The use of chemotherapy and RT in recurrent/disseminated CPC requires further study as the existing data is sparse. Information on the demographics and treatment of CPN in general can be illustrated by a single institution’s experience – see below.
Hosmann and colleagues, in a retrospective review, studied the demographics and treatment of CPNs in 36 consecutive pediatric and adult patients treated at the Medical University of Vienna between 1991 and 2016. [7] This review included 17 children, with a median age of 1.2 years, and 19 adults, with a median age of 48.5 years, in cohorts of 21 choroid plexus pappilomas, 11 atypical papillomas, and four CPCs, all of which were diagnosed in children, but none of these were ≤ 6 months of age. [7]
GTR was achieved in 91.7% of patients. A 25% tumor recurrence rate was associated with histological grading (p = 0.004), subtotal resection (p = 0.002), and tumor infiltration into brain parenchyma (p = 0.001). Adjuvant therapy was performed in 19.4% of patients, most of whom had a CPC. OS was dependent on the extent of resection (p = 0.001), tumor progression (p = 0.04), and the presence of leptomeningeal metastases (p = 0.002). [7] Hence, patients with CPC, tumor progression, and leptomeningeal metastases, such as the child we present here, were in a very high-risk category.
Hossman and colleagues concluded that GTR was the most important predictor of OS. In addition, tumor location and vascularization, along with hydrocephalus and patient age, were important determinates of perioperative morbidity. [7]
The utility of neoadjuvant or adjuvant RT and chemotherapy in CPC awaits resolution. In the future, epigenetic patterns [12], methylation profiling [13], proliferation markers (Ki-67/MIB-1) and tumor suppressor proteins (p53), [14, 15, 16], as well as age-related chromosomal alterations [17] may predict clinical outcome and provide insight into the use of neoadjuvant or adjuvant therapies.
We present here the use of ANP in the treatment of recurrent/disseminated CPC in a one-year and nine-month-old child following prior GTR of the tumor performed elsewhere. In the absence of any standardized therapy, the use of ANP avoided the negative sequalae of chemotherapy, RT, and/or second surgery. Despite PD the patient has obtained an OS of > 23.6 years. This may be due to an ANP effect on the invasiveness of the recurrent/disseminated CPC. We previously reported the case of a patient with glioblastoma (GBM) who obtained a PR with ANP and then underwent GTR of the persistent GBM. At the time of surgery there was no evidence of involvement of the underlying brain parenchyma suggesting an ANP effect on the tumor’s invasive potential. Now, > 23.6 years later, the patient is experiencing good health and showing no evidence of tumor recurrence. [18] The prolonged OS in these patients, with less than a CR, suggests that OS is the most important endpoint for clinical trials of ANP.
Antineoplaston research began in 1967, when significant deficiencies were noticed in the peptide content of the serum of patients with cancer compared with healthy persons. Initially Antineoplastons were isolated from blood and later from urine [19]. Subsequent studies of the isolated Antineoplastons demonstrated that Antineoplaston A10 and Antineoplaston AS2-1 were the most active ANPs. The chemical name of Antineoplaston A10 is 3-phenylacetylamino-2,6-piperidinedione. It consists of the cyclic form of L-glutamine connected by a peptide bond to phenylacetyl residue. When given orally, Antineoplaston A10 resists the attack of gastric enzymes. In the small intestine, under alkaline conditions, 30% is digested into phenylacetylglutamine (PG) and phenylacetylisoglutaminate (isoPG) in a ratio of approximately 4:1. The mixture of synthetic PG and isoPG in a 4:1 ratio, dissolved in sterile water constitutes Antineoplaston A10 IV injection. Further metabolism of Antineoplaston A10 results in phenylacetate (PN). Both metabolites, PG and PN, have anticancer activity. The mixture of PN and PG in a 4:1 ratio, dissolved in sterile water constitutes Antineoplaston AS2-1 IV injection. [20]
ANP’s mechanism of action differs from that of RT or cytotoxic chemotherapy. Growth of normal cells is controlled by cell cycle progression genes (oncogenes) and by cell cycle arrest genes (tumor suppressor genes). In cancer, alteration of these control genes in malignant cells favors aggressive cell proliferation. Evidence suggests that ANP affects 400 mutated genes in the malignant genome and functions as a “molecular switch” which “turns on” tumor-suppressor genes and “turns off” oncogenes [21/22]. Hence, the ntineoplastic action of ANP involves restoration of cell cycle control, induction of programmed cell death, and interference with cancer cell metabolism and nuclear transport.
Conclusion
We have presented the case of a one-year and nine-month-old female with a recurrent/disseminated CPC who despite PD has obtained an OS of greater than 23.6 years and good health following ANP, which may be due to an ANP effect on the invasiveness of the recurrent/disseminated CPC. ANP has proved to be an attractive option for patients with persistent, recurrent, disseminated, and/or metastatic brain tumors as it produces ORs and avoids the negative sequalae of chemotherapy, RT, and/or second surgery. Multiple Phase II clinical studies of ANP in a variety of low-and high-grade brain tumors under the Burzynski Research Institute’s IND # 43,742 have now been completed and numerous articles have been published [23-68]. Based on our findings, we propose a multi-institutional Phase II clinical study of ANP in CPC.
Acknowledgements
The authors express their appreciation to Carolyn Powers for preparation of the manuscript and to Ramiro Rivera, Mohamed Khan, Jennifer Pineda, and Adam Golunski for their involvement.
The authors express their appreciation to Carolyn Powers for preparation of the manuscript and to Ramiro Rivera, Mohamed Khan, Jennifer Pineda, and Adam Golunski for their involvement.
References
- Asai A, Hoffman HJ, Hendrick EB, Humphreys RP, Becker L. (1989). Primary intracranial neoplasms in the first year of life. Childs Nerv Syst 5: 230–233
- Jooma R, Hayward R. Grant N. (1984). Intracranial neoplasms during the first year of life: Analysis of one hundred consecutive cases. Neurosurgery 14: 31–41.
- Emerich DF, Skinner SJ, Borlongan CV, Vasconcellos AV. Thanos, C.G. (2005). The choroid plexus in the rise, fall and repair of the brain. Bioassays 27: 262–274.
- Wolburg H, Paulus W, Choroid plexus: Biology and pathology. (2010) Acta Neuropathol 119: 75–88.
- Torp SH, Solheim O, Skjulsvik AJ. (2022). The WHO 2021 Classification of Central Nervous System tumours: A practical update on what neurosurgeons need to know-a minireview. Acta Neurochir 164: 2453–2464.
- Wolff JE, Sajedi M, Brant R, Coppes MJ Egeler RM. (2002). Choroid plexus tumours. Br. J. Cancer 87: 1086–1091.
- Hosmann A, Hinker F, Dorfer C, Slavc I, Haberler C, Dieckmann K, Knosp E, Czech T. (2019). Management of choroid plexus tumors - an institutional experience. Acta Neurochir (Wien) 161(4): 745-754.
- Duffner PK, Kun LE, Burger PC, Horowitz ME, Cohen ME, Sanford RA; Krischer J, Mulhern RK, James H.E. Reka H.L. et al. (1995) Postoperative chemotherapy and delayed radiation in infants and very young children with choroid plexus carcinomas. The Pediatric Oncology Group - Pediatr Neurosurg 22: 189–196.
- Lin H. Leng X, Qin Ch, et al. (2019). Choroid plexus tumours on MRI: similarities and distinctions in different grades. Cancer Imaging 19: 17.
- Wen PK, Macdonald DR, Reardon DA, et al. (2010 Updated response criteria for high-grade gliomas: Response Assessment in Neuro-Oncology (RANO) working group. J Clin Oncol. 28(11): 1963-1972.
- Chinot OL, Macdonald DR, Abrey LE, Zahlman G, Kerloeguen Y, Cloughesy TF. Response Assessment Criteria for Glioblastoma: Practical Adaptation and Implementation in Clinical Trials of Antiangiogenic Therapy. (2013) Curr Neuro Neurosci Rep 13(347).
- Merino DM, Shlien A, Villani A, Pienkowska M, Mack S, Ramaswamy V, Shih D, Tatevossian R, Novokmet A, Choufani S, Dvir R, Ben-Arush M, Harris BT, Hwang EI, Lulla R, Pfister SM, Achatz MI, Jabado N, Finlay JL, Weksberg R, Bouffet E, Hawkins C, Taylor MD, Tabori U, Ellison DW, Gilbertson RJ, Malkin D. (2015) Molecular characterization of choroid plexus tumors reveals novel clinically relevant subgroups. Clin Cancer Res.21: 184–192.
- Thomas C, Sill M, Ruland V, Witten A, Hartung S, Kordes U, Jeibmann A, Beschorner R, Keyvani K, Bergmann M, Mittelbronn M, Pietsch T, Felsberg J, Monoranu CM, Varlet P, Hauser P, Olar A, Grundy RG, Wolff JE, Korshunov A, Jones DT, Bewerunge-Hudler M, Hovestadt V, von Deimling A, Pfister SM, Paulus W, Capper D, Hasselblatt M. (2016) Methylation profiling of choroid plexus tumors reveals 3 clinically distinct subgroups. Neuro-Oncology 18: 790–796.
- Wrede B, Hasselblatt M, Peters O, Thall PF, Kutluk T, Moghrabi A, Mahajan A, Rutkowski S, Diez B, Wang X, Pietsch T, Kortmann RD, Paulus W, Jeibmann A, Wolff JE. (2009). Atypical choroid plexus papilloma: clinical experience in the CPT-SIOP-2000 study. J Neuro-Oncol 95: 383–392.
- Tabori U, Shlien A, Baskin B, Levitt S, Ray P, Alon N, Hawkins C, Bouffet E, Pienkowska M, Lafay-Cousin L, Gozali A, Zhukova N, Shane L, Gonzalez I, Finlay J, Malkin D. (2009). TP53 alterations determine clinical subgroups and survival of patients with choroid plexus tumors. J Clin Oncol 28: 1995–2001.
- El-Deiry WS. (2003). The role of p53 in chemosensitivity and radiosensitivity. Oncogene. 22: 7486–7495.
- Ruland V, Hartung S, Kordes U, Wolff JE, Paulus W, Hasselblatt M. (2014). Choroid plexus carcinomas are characterized by complex chromosomal alterations related to patient age and prognosis. Genes Chromosomes Cancer 53: 373–380.
- Burzynski S, Burzynski G, Janicki T, Beenken, S. (2022). Newly diagnosed Glioblastoma: Partial Response and > 27 Years Overall Survival in a 37-Year-Old Male Treated with Antineoplastons. Recent Adv Clin Trials 1(2): 1-7.
- Burzynski SR. Antineoplastons: Biochemical defense against cancer. (1976). Physiol Chem Phys 8: 275-279.
- Burzynski SR (1986). Synthetic antineoplastons and analogs: Drugs of the future. 11:679-688.
- Burzynski SR, Patil S. (2014). The effect of Antineoplaston A10 and AS2-1 and metabolites of sodium phenylbutyrate on gene expression in glioblastoma multiforme. J Cancer Ther 5: 929-945.
- Burzynski SR, Janicki T, Burzynski G. (2015). Comprehensive genomic profiling of recurrent classic glioblastoma in a patient surviving eleven years following antineoplaston therapy Cancer Clin Oncol 4(2): 41-52.
- Burzynski SR, Conde AB, Peters A, Saling B, Ellithorpe R, Daugherty JP, Nacht CH. (1999). A Retrospective Study of Antineoplastons A10 and AS2-1 in Primary Brain Tumors. Clin Drug Invest 18, 1-10.
- Burzynski SR, Weaver RA, Lewy RI, Janicki TJ, Jurida GF, et al. (2004). Phase II study of Antineoplaston A10 and AS2-1 in children with recurrent and progressive multicentric glioma: A preliminary Report Drugs R D 5: 315-326.
- Burzynski SR, Lewy RI, Weaver R, Janicki T, Jurida G, Khan M, Larisma CB, Paszkowiak J, Szymkowski B. (2004) Long-term survival and complete response of a patient with recurrent diffuse intrinsic brain stem glioblastoma multiforme. Integ Cancer Ther 3: 257-261.
- Burzynski SR, Weaver R, Bestak M, Janicki T, Szymkowski B, Jurida G, Khan M, Dolgopolov, V. (2004). Treatment of primitive neuroectodermal tumors (PNET) with antineoplastons A10 and AS2-1 (ANP): Preliminary results of phase II studies. Neuro Oncol. 6: 428.
- Burzynski SR, Weaver R, Bestak M, Janicki T, Jurida G., Szymkowski B, Khan M, Dolgopolov V. (2004). Phase II studies of antineoplastons A10 and AS2-1 (ANP) in children with atypical teratoid/rhabdoid tumors (AT/RT) of the central nervous system: A preliminary report. Neuro Oncol 6; 427.
- Burzynski SR, Weaver RA, Janicki, T, Szymkowski B, Jurida G, Khan M, Dolgopolov V. (2005). Long-term survival of high-risk pediatric patients with primitive neuroectodermal tumors treated with
- Antineoplastons A10 and AS2-1. Integ Cancer Ther 4(2): 168-177.
- Burzynski SR. (2006). Targeted Therapy for Brain Tumors. In: Yang AV, editor. Brain Cancer Therapy and Surgical Interventions. Nova Science Publishers, Inc, New York.
- Burzynski SR, Janicki, TJ, Weaver RA, Burzynski B. (2006). Targeted therapy with Antineoplastons A10 and AS2-1 of high grade, recurrent, and progressive brainstem glioma. Integ Cancer Ther 5(1), 40-47.
- Burzynski SR. (2006) Treatments for astrocytic tumors in children: Current and emerging strategies. Ped Drugs. 8: 167-168.
- Burzynski SR. (2007). Recent clinical trials in diffuse intrinsic brainstem glioma. Cancer Ther. 5: 379- 390.
- Burzynski SR, Burzynski GS, Janicki TJ. (2004) Recurrent glioblastoma multiforme: A strategy for long-term survival. J Cancer Ther. 5: 957-976.
- Burzynski SR, Janicki TJ, Burzynski GS, Marszalek A. (2014). A phase II study of antineoplastons A10 and AS2-1 in children with high-grade glioma: Final report (Protocol BT-06) and review of recent trials. J Cancer Ther 5: 565-577.
- Burzynski SR, Janicki TJ, Burzynski GS. (2014) A phase II study of antineoplastons A10 and AS2-1 in adult patients with recurrent glioblastoma multiforme: Final report (Protocol BT-21). J Cancer Ther 5: 946-956.
- Burzynski SR, Janicki TJ, Burzynski, GS, Marszalek A, Brookman S. (2014) A phase II study of antineoplastons A10 and AS2-1 in children with recurrent, refractory or progressive primary brain tumors: Final report (Protocol BT-22). J Cancer Ther 5: 977-988.
- Burzynski SR, Janicki TJ, Burzynski GS, Brookman S. (2014). Preliminary findings on the use of targeted therapy with pazopanib and other agents in combination with sodium phenylbutyrate in the treatment of glioblastoma multiforme. J Cancer Ther 5: 1423-1437.
- Burzynski GS, Janicki TJ, Marszalek A. Long-term survival (> 20 years) of a child with brainstem glioma treated with antineoplastons A10 and AS2-1: A case report. (2014). Neuro Oncol. 11:16.
- Burzynski SR, Janicki TJ, Burzynski GS, Marszalek. (2014) The response and survival of children with recurrent diffuse intrinsic pontine glioma based on phase II study of antineoplastons A10 and AS2-1 in patients with brainstem glioma. Childs Nerv Syst 30: 2051-2061.
- Burzynski S, Janicki T, Burzynski G, Marszalek A. (2014) Long-term survival (> 13 years) in a child with recurrent diffuse pontine gliosarcoma: A case report. J Ped Hematol Oncol 36: 433-439.
- Burzynski SR, Janicki T, Burzynski G. (2015) A phase II study of Antineoplastons A10 and AS in adult patients with primary brain tumors: Final report (Protocol BT-09), J Cancer Ther 6: 1063-1074.
- Burzynski SR, Burzynski G, Janicki J, Marszalek A. (2015). Complete response, and Long-term survival (> 20 years) in a child with tectal glioma: A case report. Pediatr Neurosurg 50: 99-103.
- Burzynski SR, Janicki TJ, Burzynski GS, Marszalek A. (2015). A Phase II Study of Antineoplastons A10 and AS2-1 in adult patients with newly diagnosed anaplastic astrocytoma: Final report (Protocol BT-08). Cancer Clin Oncol 4: 28-38.
- Burzynski SR, Janicki TJ, Burzynski G. (2015). A phase II study of Antineoplastons A10 and AS2-1 injections in adult patients with recurrent anaplastic astrocytoma: Final report (Protocol BT-15). Cancer Clin Oncol 442: 13-23.
- Burzynski SR, Burzynski GS, Marszalek A, Janicki J, Martinez-Canca J. (2015). Long-term survival (greater than 20 years), complete response, and normal childhood development in medulloblastoma treated with Antineoplastons A10 and AS2-1. J Neurol Stroke 2(3): 00054.
- Burzynski SR, Burzynski GS, Marszalek A, Janicki TJ, Martinez-Canca JF. (2005). Long-term survival over 21 years and pathologically confirmed complete response in pediatric anaplastic astrocytoma: A case report. J Neurol Stroke. 2(6): 00072.
- Burzynski SR, Burzynski GS, Brookman S. (2015). A case of sustained objective response of recurrent/progressive diffuse intrinsic pontine glioma with phenylbutyrate and targeted agents. J Cancer Ther 6: 40-44.
- Burzynski SR, Janicki, T, Burzynski G, Marszalek A. (2015). A phase II study of antineoplastons A10 and AS2-1 in patients with brainstem gliomas: The report on non-diffuse intrinsic pontine glioma (Protocol BT-11). J Cancer Ther 6: 334-344.
- Burzynski S, Janicki TJ, Burzynski GS. (2016). Primary CNS tumors and leptomeningeal, disseminated, and/or multicentric disease in children treated in phase II studies with antineoplastons A10 and AS2-1. Cancer Clin Oncol 5(2): 38-48.
- Burzynski SR, Janicki TJ, Burzynski GS. (2016). A phase II study of antineoplastons A10 and AS2-1 in children with low-grade astrocytomas: Final report (Protocol BT-13). J Cancer Ther 7(12): 837-850.
- Burzynski SR, Janicki TJ, Burzynski GS, Marszalek A. (2017). A phase II study of Antineoplastons A10 and AS2-1 in children with brain tumors: Final report (Protocol BT-10). J Cancer Ther 8: 173-187.
- Burzynski SR, Janicki T, Beenken, S. (2019). Treatment of recurrent glioblastoma multiforme (rGBM) with Antineoplaston AS2-1 in combination with targeted therapy. Cancer Clin Oncol 8: 1-15.
- Burzynski, SR, Janicki, T, Burzynski, GS, Beenken, S. (2021). Long-term survival (27.7 years) following IV Antineoplaston Therapy (ANP) in a 36-year-old-female with a progressive diffuse intrinsic pontine glioma (DIPG). Int J Radiol Imaging Technol 7: 073-078.
- Burzynski, SR, Burzynski, GS, Janicki, T, Beenken S. (2021). Long-term survival (23 years) in a 26-year-old male after Antineoplaston therapy for a progressive, diffuse intrinsic pontine glioma: A case report. Int J Brain Disorder Treat 6: 038-044.
- Burzynski, SR, Janicki T, Burzynski GS, Beenken S. (2021). Resolution of clinical signs, a complete response, and long-term survival (23 Years) in a 3 and ½ month female with a newly diagnosed diffuse intrinsic pontine glioma treated with antineoplastons. Biomed Res Clin Prac 6: 1-6.
- Burzynski, SR, Janicki T, Burzynski GS, Beenken S. (2021). Diffuse intrinsic pontine glioma in an 11-year-old female treated with antineoplastons: Complete response and > 25-year survival. Pediatr Neonatal Med 1(2):1-5.
- Burzynski SR, Janicki T, Burzynski GS, Beenken S. (2022) A 25-year-old female with diffuse intrinsic pontine glioma surviving for more than nine years following treatment with antineoplastons. Int J Clin Oncol Cancer Res 7:1-7.
- Burzynski SR, Burzynski GS, Janicki T, Beenken S. (2022). Twenty-two-year survival in a 15-year-old female with a recurrent posterior fossa ependymoma treated with antineoplastons. Oncol Clin Res 3(1): 99-105.
- Burzynski S, Burzynski G, Janicki T, Beenken, S. (2022). Newly diagnosed Multicentric Pilocytic Astrocytoma: Complete Response and > 22 Years Survival in a Six Year and Nine-month-old Female Treated with Antineoplastons. Internat J Clin Oncol and Cancer Res 7 (3): 76-82.
- Burzynski S, Burzynski G, Janicki T, Beenken, S. (2022) Recurrent and progressive ganglioglioma in an 11-year-old male treated with antineoplastons: Partial response with more than nine years and nine months survival and complete resolution of clinical symptoms/signs. Biomed Res J 37: 1-13.
- Burzynski S, Burzynski G, Janicki T, Beenken, S. (2022). Outcomes in Four Children with Persistent, Recurrent, and Progressive Gangliogliomas Treated in Phase II Studies with Antineoplastons A10 and AS2-1. Neurol Neurosci 3(3): 1-9.
- Burzynski S, Burzynski G, Janicki T, Beenken, S. (2022). Recurrent/Persistent Glioblastoma: Complete Response and 24 Years Disease-Free-Survival in a 45-Year-Old Female Treated with Antineoplastons. Cancer Studies Therap 7(3): 1-6.
- Burzynski SR, Burzynski GS, Janicki, TJ, Beenken, SW. (2023). Persistent Pineoblastoma: Complete Response and > 26 Years Overall Survival in a Ten-month-old Female Treated with Antineoplastons. Biomed Res Clin Prac 7: 1-5.
- Burzynski S, Burzynski G, Janicki T, Beenken S. (2023). Newly diagnosed Anaplastic Astrocytoma:
> 23 Year Survival in a 31-Year and 11-month-Old Female treated with Antineoplastons. Neurol Neurosci 4(2): 1-6. - Burzynski S, Burzynski G, Janicki T, Beenken S. (2023). Inoperable Optic Pathway Glioma: A Seven-Year-Old Male with > 35 Years Overall Survival Following Treatment with Antineoplastons. Ej-clinicmed.org 4(5): 9-14.
- Burzynski S, Burzynski G, Janicki T, Beenken S. (2023) Recurrent Medulloblastoma: Complete Response and > than 21 Years and Five Months Overall Survival in a One-Year and Seven-Month-Old Male Treated with Antineoplastons. J Oncol Res Rev Rep 4(4): 3-6.
- Burzynski S, Burzynski G, Janicki T, Beenken S. (2024). Recurrent Pilocytic Astrocytoma: Treatment with Antineoplastons, Complete Response, and > 27 Years Overall Survival. Recent Adv Clin Trials 4(1): 1-7.
Citation: Stanislaw R. Burzynski, Gregory S. Burzynski, Tomasz Janicki and Samuel Beenken. (2024). Recurrent/Disseminated Choroid Plexus Carcinoma: Overall Survival of > 23.6 Years in a One-Year and Nine-Month-Old Female Treated with Antineoplastons. Journal of Medical Research and Case Reports 6(1). DOI: 10.5281/zenodo.11387006
Copyright: © 2024 Stanislaw R. Burzynski. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

