Case Report
Volume 5 Issue 1 - 2023
Successful Eradication of Rituximab-Refractory Post-Transplant Lymphoproliferative Disorder after Second Haplo-identical Allogeneic Hematopoietic Stem Cell Transplantation
Chief of the Department of Stem Cell Transplantation and Cellular Therapy, Sr Director of Hematopoietic Stem Cell Transplantation Multidisciplinary Team, Koo Foundation Sun Yet-Sen Cancer Center, Taipei, Taiwan (R.O.C.), 6F, No.125, Lih-Der Road, Bei-Tou District, Taipei City 11259, Taiwan (R.O.C.)
*Corresponding Author: Tran-Der Tan, Chief of the Department of Stem Cell Transplantation and Cellular Therapy, Sr Director of Hematopoietic Stem Cell Transplantation Multidisciplinary Team, Koo Foundation Sun Yet-Sen Cancer Center, Taipei, Taiwan (R.O.C.), 6F, No.125, Lih-Der Road, Bei-Tou District, Taipei City 11259, Taiwan (R.O.C.)
Received: December 24, 2022; Published: January 03, 2023
Abstract
Epstein-Barr virus (EBV)-related post-transplant lymphoproliferative disorder (PTLD) is a potentially fatal disorder arising after a solid organ or hematopoietic stem cell transplant (HSCT). The treatment includes reducing immune suppression, rituximab, and chemotherapy. We presented a female patient with very severe aplastic anemia (VSAA) with initial graft dysfunction. Graft failure occurred two months after haploidentical allogeneic HSCT, with no response to salvage cyclosporine plus eltrombopag. EBV-related PTLD occurred six months after the first haplo-transplant, as shown by high EBV PCR. The patient initially responded to 6 shots of weekly rituximab. Then progression to the ultrahigh level of EBV DNAemia was observed with the presence of intra-hepatic PTLD that was refractory to rituximab. This patient subsequently underwent a second haploidentical allogeneic transplant from the same donor with a megadose of stem cell number plus alkylating agent containing conditioning regimen. The results were successful hematopoietic engraftment on day +15, clearance of EBV DNAemia in one month, and eradication of intra-hepatic EBV-PTLD. In conclusion, EBV-related PTLD refractory to rituximab alone is still sensitive to rituximab plus preparative chemotherapy regimen during subsequent allogeneic HSCT. Therefore, it is still feasible to perform allo-transplant for transplant-eligible patients despite the presence of rituximab-refractory EBV DNAemia and PTLD.
Keywords: Epstein-Barr virus (EBV); Post-transplant lymphoproliferative disorder (PTLD); Rituximab-refractory; Aplastic anemia; Haploidentical allogeneic hematopoietic stem cell transplant
Case Presentation
A 29-year-old kindergarten teacher is a patient with very severe aplastic anemia with the presentation of dizziness and fatigue in October 2019. She was found to have pancytopenia with hemoglobin dropped from 6.2 to 4.3 gm/dl, platelet 27,000 /ul, and WBC 3,300/ul, with less than 1% of bone marrow hematopoietic tissue two months later. After one month, her peripheral blood absolute neutrophil count dropped to below 500/ul, and below 200/ul two months later. The patient initially underwent transfusion with two units of packed cells monthly, then every 2 to 3 weeks, and platelet weekly. She underwent haploidentical allogeneic HSCT from her younger brother, who was HLA matched in 7 out of 10 loci on Apr 15, 2020, three months after the diagnosis of severe aplastic anemia, with a conditioning regimen [1] including rabbit anti-thymocyte globulin 0.5 mg/kg on day -9 and 2 mg/kg on days -8 and -7. Fludarabine was administered 30 mg/m2 intravenously daily for five days from day -6 to day -2, cyclophosphamide was given 14.5 mg/kg intravenously daily for two days from day -6 to day -5, and total body irradiation in a single dose of 2 Gy on day -1. The peripheral blood hematopoietic stem cell with 8 x 10e6/kg CD34+ cell infusion on day 0. Post-transplant cyclophosphamide (PTCY) as the prophylaxis of acute graft-versus-host disease (GVHD), was administered at 50 mg/kg/day intravenously on days +3 and +4 after transplantation. Granulocyte colony-stimulating factor was given subcutaneously starting on days +5 at 5 mcg/kg/day until the absolute neutrophil count was greater than 1.5 x 10e3/ul for three days. Cyclosporine from day 5 through day 180, and mycophenolate mofetil from day 5 through 35. Pre-transplant screening of donor-specific antibodies was negative.
After haplo-transplant, the patient got neutrophil engrafted on +23 days. However, neutrophils dropped to below 1,000/ul once GCSF was discontinued, and intermittent use of GCSF after that was aimed at treating poor graft function. Unfortunately, until two months after the transplant, the patient could not maintain an absolute neutrophil count above 500/ul even under daily GCSF and the administration of twice donor lymphocyte infusion (DLI). Pancytopenia then occurred due to graft failure. After that, we tried combined cyclosporine and eltrombopag treatment between June and December 2020 (+2 ~ +8 months post-transplant), but no response.
Peripheral blood mononuclear cell EBV PCR elevating from 23,709 to 68,100 IU/ug of genomic DNA occurred six months after the first transplant. The patient initially responded to four weekly doses of rituximab 375 mg/m2, and elevated EBV PCR recurred two weeks after rituximab. There was still a response after rituximab was resumed; however, there was no more response to the subsequent rituximab treatment two months later. Both plasma and PBMN cell EBV PCR elevated to 235,767 IU/ml and 218,411 IU/ug of genomic DNA, respectively, before the second haplo-transplant. At the same time, we found scattered intrahepatic nodules, which proved to be EBV-related PTLD after a liver biopsy.
Therefore, we performed the second haploidentical allogeneic hematopoietic stem cell transplantation from the same donor with a reduced intensity conditioning (RIC) regimen. Alkylating agent melphalan with fludarabine plus TBI 2 Gy (FluMel + TBI 2 Gy), plus a high dose of rituximab 1000 mg in January 2021. Haplo-stem cell infusion with CD34+ cell 16 x 10e6/kg on Jan 26, 2021, followed by post-transplant high dose cyclophosphamide 35 mg/kg on days +3 and +4. Neutrophil and platelet were engrafted on +14 and +17 days, respectively.
Plasma and peripheral blood mononuclear cell EBV dropped dramatically from 235,767 IU/ml and 218,411 IU/ug on day -1 to 1,177 IU/ml and 1,747 IU/ug, respectively, on day +6 and then declined until they were undetected on day +34 after second haplo-transplant (table 1 and figure 1). Prior "intrahepatic PTLD nodules" biopsy performed on +20 days showed total necrotic tissues except one cell stained positive for EBV, indicating the prior intrahepatic PTLD became necrotic and resolved in the subsequent months (Figure 2). At the time of the report in December 2022, the patient got well and was living with persistent PTLD-free conditions in the liver and undetected EBV PCR in plasma and PBMN cells.
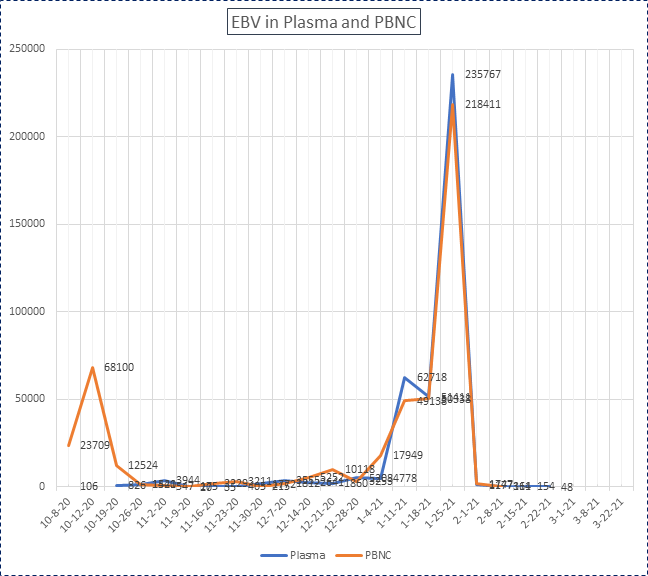
Figure 1: EBV in plasma and PBNC level (IU/ml and IU/ug of genomic DNA, respectively). The first haploidentical allogeneic hematopoietic stem cell transplantation was performed on Apr 15, 2020, and the second haploidentical HSCT on Jan 26, 2021.
2021/08/23
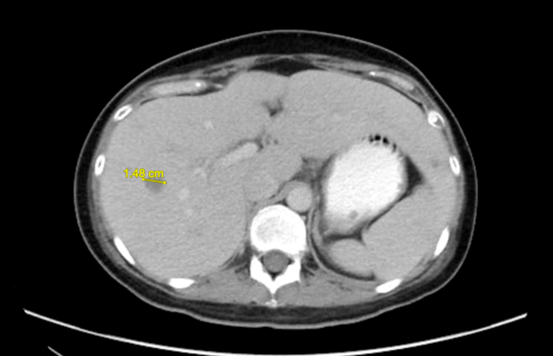
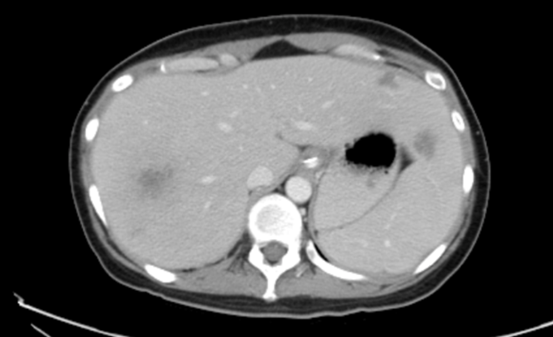
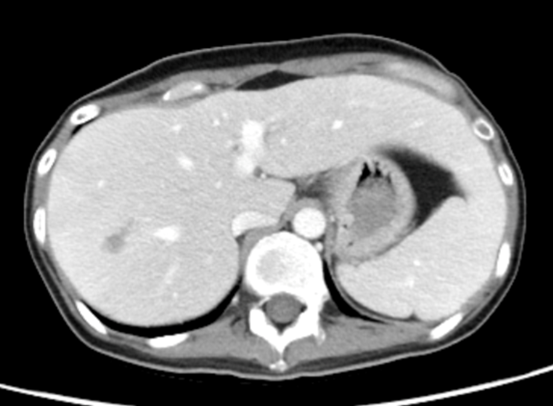
Figure 2: Intrahepatic infiltrative lesions were seen in (A) 4 weeks before the second haplo-transplant; (B) 3 weeks after the second haplo-transplant; (C) 7 months after the second haplo-transplant.
Figure 2: Intrahepatic infiltrative lesions were seen in (A) 4 weeks before the second haplo-transplant; (B) 3 weeks after the second haplo-transplant; (C) 7 months after the second haplo-transplant.
Discussion
Treatment of severe or very severe aplastic anemia includes allogeneic hematopoietic stem cell transplantation (HSCT) and immune suppression therapy (IST). For patients older than 50, IST, especially triple therapy (ATG, cyclosporine, and eltrombopag), maybe a preferred option due to increased transplant-related morbidity and mortality in this age group. However, even higher response rates achieved in triple therapy -- most of them partial rather than complete response -- and disease relapse, clonal evolution, and evolution to myeloid malignancy are a matter of concern [2].
For those younger than 50 years of age and transplant-eligible, allogeneic hematopoietic stem cell transplantation is also an option in the event of IST failure. Since there was no definitive comparison of outcome between frontline and salvage allo-transplant, allogeneic hematopoietic stem cell transplantation would be a preferred choice. We make this recommendation because of the long-term persistent risk of disease relapse, secondary myelodysplastic syndrome, and acute myeloid leukemia with the use of nontransplant IST for patients with aplastic anemia [3].
However, transplant-related morbidity and mortality and post-transplant acute and chronic graft versus host disease (GVHD) would also be sources of concern. On account of allo-HSCT, if a patient cannot reach a matched related or matched unrelated donor. We have to choose an alternative donor, e.g., mismatched unrelated, or haploidentical transplant. Meanwhile, suppose a severe or very severe aplastic anemia patient does not have matched or has one locus mismatched unrelated donor. In that case, a haploidentical donor may be a better choice since there would be a far longer time to reach matched unrelated donors.
In this patient, we chose allogeneic hematopoietic stem cells from a haploidentical donor (3-loci mismatched) as frontline treatment because the patient was relatively young and had no matched unrelated donor. The preparative regimen was according to the Johns Hopkins protocol (ATG-FluCy + TBI 2 Gy), which was effective either in the frontline [4] or salvage transplant setting [1]. However, poor graft function was found one month after haplo-transplant, and graft failure occurred in this patient two months later. According to that series, there was an 11% graft failure rate, with one in 20 in the salvage transplant setting and 3 in 17 in the frontline setting suffering relapsed SAA; two patients passed away in 4 and 8 months, respectively. In their limited number of patients' experience, overcoming graft rejection seemed possible by an escalation of TBI dose from 2 Gy to 4 Gy. However, there was no specific preferred treatment option after graft failure, and a second haplo-transplant was still the only curable treatment. We had tried combined cyclosporine and eltrombopag [5] after graft failure, although results derived from the limited experience have shown a response rate of up to 55% [6,7]. After that, we decided to perform the second haplo-transplant from the same donor with a megadose of stem cells (16 x 10e6/kg) and alkylating agent containing a preparative regimen (FluMel + TBI 2Gy) to overcome graft rejection in this patient. It worked with neutrophil engrafted on +14 days, and the situation continued until 18 months after the second haplo-transplant.
EBV-related PTLD is a potentially fatal disorder arising after a solid organ transplant or HSCT [8]. In the post-HSCT setting, especially for the high-risk population of patients, e.g., T cell depletion of graft (including ATG use), HLA mismatch, and severe GVHD, EBV PCR should be monitored periodically after hematopoietic engraftment (e.g., weekly to every two weeks after engraftment until 100 days after transplant). The management principle includes the deduction of immune suppression followed by rituximab, and pre-emptive therapy is justified in an allogeneic HSCT setting. Chemotherapy such as CHOP or a CHOP-like regimen is viable for treating large B cell lymphoma but is usually too toxic for post-allotransplant patients. Adoptive immunotherapy is a promising therapeutic modality in pre-emptive settings and as treatment of established EBV-positive PTLD. However, the use is still restricted because of labor-intensive procedures, reimbursement issues, and availability issues [9]. Rituximab-based pre-emptive treatment can prevent EBV-DNAemia from developing into EBV-PTLDs. The current treatment strategies for probable and proven EBV-PTLDs include immunosuppressants withdrawal, rituximab, adoptive cell therapy (DLI or EBV-CTLs), chemotherapy, radiotherapy, and surgery, among which rituximab plus/minus chemotherapy and immunosuppressants withdrawal are the mainstays [10].
In our patient with EBV DNAemia 6 months after the first haplo-transplant, she responded to rituximab initially until three months later as EBV DNAemia persisted and then progressed to DNAemia refractory to rituximab. She subsequently had intrahepatic PTLD. For her, it is not easy to give chemotherapy -- because of pancytopenia -- until the preparative chemotherapy regimen (fludarabine and melphalan) and high dose rituximab in the second haplo-transplant. Her EBV DNAemia dramatically dropped to below 0.5% post-transplant +6 days and then declined gradually until undetected on +34 days and beyond 18 months. Besides, the intra-hepatic tumor, which resulted from EBV-related PTLD, turned out to be necrotic four weeks after the transplant and was resolved.
What is the reason for the successful eradication of EBV DNAemia and intrahepatic PTLD in this patient? There were three main factors:
- The resumption of high-dose rituximab in addition to the preparative chemotherapy regimen
- Allogeneic donor lymphocytes containing peripheral blood hematopoietic stem cell infusion, which was composed of cytotoxic or even EBV-directed T cells against EBV
- Hematopoietic reconstitution in time and immune reconstitution
With rituximab administered as a single agent at a dose of 375 mg/m2 of body surface area weekly for four weeks, the overall response rates after reduced immunosuppression and rituximab therapy are 44 to 79%, with complete remission rates of 20 to 55% [11].
Immuno-chemotherapy is indicated in patients with B-cell PTLD who have not had a response to reduced immunosuppression and rituximab administered as a single agent [11]. When our patient underwent a second haploidentical allogeneic HSCT, we gave her high-dose rituximab 1000 mg in one shot plus fludarabine 150 mg/m2 plus melphalan 120 mg/m2, and post-transplant high-dose cyclophosphamide in reduced dose with 35 mg/kg/d on +3 and +4 days. All three chemotherapy treatments were effective for B cell lymphoma, and the additive effect of high-dose rituximab could help explain why the EBV-PCR dropped to less than 0.5% in one week.
Besides, in the hematopoietic stem cell infusion, there were megadoses of stem cells, 16 million/kg of body weight of the patient. The donor had EBV-specific antibodies, and many EBV-specific cytotoxic lymphocytes were also infused. EBV-specific cytotoxic lymphocytes (CTLs) can induce a solid EBV-specific cellular immune response [12]. A strategy of adoptive immunotherapy, using donor lymphocyte infusion, was first described for treating PTLD after allogeneic HSCT, which generally arises from donor cells, unlike PTLD in recipients of solid-organ transplants. However, the administration of donor lymphocyte infusion was associated with a high risk of graft-versus-host disease [13]. In the past decade, expanded EBV-specific CTLs have been as part of prophylactic, preemptive, and therapeutic strategies with autologous CTLs (in the case of recipient-derived PTLD) and allogeneic CTLs (isolated from the donor or a bank of partially HLA-matched doors) [14]. However, the latter procedures are labor-intensive and unavailable at our institute.
Finally, this patient achieved neutrophil and platelet engraftment post-transplant +14 and +17 days, respectively. In our institute's experience, the timing of neutrophil engrafted in haploidentical HSCT is usually between +19 and +22 days with a stem cell count below 10 million/kg of the patient's body weight. However, for higher than ten million/kg stem cell dose, the engrafted time could be earlier, before +15 days [15]. Later, the early recovery of NK and T cells as immune reconstitution could play a role in treating and eradicating EBV.
Conclusion
EBV-related post-transplant lymphoproliferative disorder refractory to rituximab alone is still sensitive to rituximab plus chemotherapy containing preparative regimen during subsequent allogeneic hematopoietic stem cell transplantation, and the coming donor-derived CD3 lymphocytes and post-transplant immune reconstitution may have functions against EBV. That said, it is still feasible to perform allogeneic transplantation for transplant-eligible patients with rituximab-refractory EBV DNAemia and the presence of PTLD.
Acknowledgments
We thank the patient and her family to support her to pass the treatment and thank for our nursing practitioner Ms Chou Wan-Hua and transplant nursing staff to take care this patient.
We thank the patient and her family to support her to pass the treatment and thank for our nursing practitioner Ms Chou Wan-Hua and transplant nursing staff to take care this patient.
Conflict of Interest
Ethics approval and consent to participate: This study is a standard treatment option with no ethical issues.
Ethics approval and consent to participate: This study is a standard treatment option with no ethical issues.
Consent for publication: This is the first time for publication; this report has been presented at a Taiwan Society of Hematology meeting previously.
Availability of data and materials: All the data and materials are from standard treatment options at our hospital.
Competing interests: No competing interests.
References
- DeZern AE, Zahurak M, Symons H, Cooke K, Jones RJ, and Brodsky A. (2017). Alternative donor transplantation with high-dose post-transplantation cyclophosphamide for refractory severe aplastic anemia. Biol Blood Marrow Transplant. 23(3): 498-504.
- Townsley DM, Scheinberg P, Winkler T, Desmond R, Dumitriu B, Rios O, Weinstein B, Valdez J, Lotter J, Feng X, Desierto M, Leuva H, Bevans M, Wu C, Larochelle A, Calvo KR, Dunbar CE, Young NS. (2017). Eltrombopag Added to Standard Immunosuppression for Aplastic Anemia. N Engl J Med.;376(16):1540-1550.
- Georges GE, Doney K, and Storb R. (2018). Severe aplastic anemia: allogeneic bone marrow transplantation as first-line treatment. Blood Advances 2(15): 2020-2028.
- DeZern A, Aahurak ML, Symons HJ, Cooke KR, Rosner GL, et al. (2020). Haploidentical BMT for severe aplastic anemia with intensive GVHD prophylaxis including posttransplant cyclophosphamide. Blood Advances 4(8): 1770-1779.
- Scheinberg P. (2018). Activity of eltrombopag in severe aplastic anemia. Blood Advances 2(21): 3054-3062.
- Marotta S, Marano L, Ricci P, Cacace F, Frieri C, Simeone L, Trastulli F, Vitiello S, Cardano F, Pane F, Risitano AM. (2019). Eltrombopag for post-transplant cytopenias due to poor graft function. Bone Marrow Transplant. Aug;54(8):1346-1353.
- Halahleh, K., Gale, R.P., Da’na, W. et al. (2021). Therapy of posttransplant poor graft function with eltrombopag. Bone Marrow Transplant 56, 4–6.
- Dierickx D, Tousseyn T, and Gheysens O. (2005). How I treat posttransplant lymphoproliferative disorders. Blood. 126(20): 2274-2283.
- Dierickx D and Vergote V. (2019). Management of post-transplant lymphoproliferative disorders. HemaSphere Education Update in Hematology Book 3(S2): 74-77.
- Liu L, Zhang X, Feng Sizhou. (2018). Epstein-Barr virus-related post-transplantation lymphoproliferative disorders after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 24: 1341-1349.
- Trappe RU, Dierickx D, Zimmermann H, Morschhauser F, Mollee P, Zaucha JM, Dreyling MH, Dührsen U, Reinke P, Verhoef G, Subklewe M, Hüttmann A, Tousseyn T, Salles G, Kliem V, Hauser IA, Tarella C, Van Den Neste E, Gheysens O, Anagnostopoulos I, Leblond V, Riess H, Choquet S. (2017). Response to Rituximab Induction Is a Predictive Marker in B-Cell Post-Transplant Lymphoproliferative Disorder and Allows Successful Stratification into Rituximab or R-CHOP Consolidation in an International, Prospective, Multicenter Phase II Trial. J Clin Oncol. Feb 10;35(5):536-543.
- Thorley-Lawson DA, Gross A, Persistence of the Epstein-Barr virus and the origins of associated lymphomas. N Engl J Med 2004; 350: 1328-37.
- Papadopoulos EB, Ladanyi M, Emanuel D, Mackinnon S, Boulad F, Carabasi MH, Castro-Malaspina H, Childs BH, Gillio AP, Small TN, et al. (1994). Infusions of donor leukocytes to treat Epstein-Barr virus-associated lymphoproliferative disorders after allogeneic bone marrow transplantation. N Engl J Med. Apr 28;330(17):1185-91.
- Anna Merlo, Riccardo Turrini, Riccardo Dolcetti, Debora Martorelli, Elena Muraro, Patrizia Comoli, Antonio Rosato. (2010). The interplay between Epstein-Barr virus and the immune system: a rationale for adoptive cell therapy of EBV-related disorders. Haematologica 95(10):1769-1777.
- Chiou LW, Tan TD. Higher stem cell doses could achieve earlier engraftment of hematopoiesis in haploidentical allogeneic hematopoietic stem cell transplantation—annual Meeting of Hematology Society of Taiwan and Taiwan Society of Blood and Marrow Transplantation 2021.
Citation: Tran-Der Tan. (2023). Successful Eradication of Rituximab-Refractory Post-Transplant Lymphoproliferative Disorder after Second Haplo-identical Allogeneic Hematopoietic Stem Cell Transplantation. Journal of Medical Research and Case Reports 5(1).
Copyright: © 2023 Tran-Der Tan. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.