Research Article
Volume 3 Issue 2 - 2021
Clinical Practice Survey Regarding Management of Apremilast-Related Diarrhoeas’ in Patients with Psoriasis
1194 avenue Rubillard, 72100 Le Mans. Dermatology, General Hospital, Le Mans, France
2194 avenue Rubillard, 72100 Le Mans. Nephrology, General Hospital, Le Mans, France
3194 avenue Rubillard, 72100 Le Mans. Hepato-gastroenterology, General Hospital, Le Mans, France
4University of Torino, Italy
2194 avenue Rubillard, 72100 Le Mans. Nephrology, General Hospital, Le Mans, France
3194 avenue Rubillard, 72100 Le Mans. Hepato-gastroenterology, General Hospital, Le Mans, France
4University of Torino, Italy
*Corresponding Author: Chloé Venuto, 194 Avenue Rubillard, 72100 Le Mans. Dermatology, General Hospital, Le Mans, France.
Received: December 20, 2021; Published: December 30, 2021
Abstract
Background: Psoriasis affects about 2% of population. Apremilast is the first oral phosphodiesterase 4 (PDEA4) inhibitor marketed in Europe in 2013 and available in France since 2016. Even though this medication is considered to be safe, side effects are frequent with diarrhoea being the most common. No official guideline on apremilast-induced diarrhoea management is currently available. In order to offer clear and simple recommendations to manage diarrhoeas caused by apremilast, we reviewed the literature and surveyed the current practices of dermatologists in France.
Methods: We sent via a French network (ResoPso) a questionnaire to hospital and private practice dermatologists.
Results: 19% of the dermatologists offer a symptomatic treatment, 10.2% non-pharmacologic interventions, 64.6% a combination of the two. 6.1% of the dermatologist’s definitively stop apremilast. 74% of the dermatologists prescribe racecadotril as a first-line symptomatic treatment.
Discussion: Diarrhoea due to apremilast affects 15% of patients. The first action to manage diarrhoea is to grade its severity and treat its complications. The first treatment step are dietary and hygienic advice, followed by the prescription of racecadotril. An alternative approach involves the reduction of the dose of apremilast or it’s temporarily discontinuation. After discontinuation and reversal of diarrhoea, the treatment can be restarted with increasing doses.
Keywords: Psoriasis; Therapeutic; Apremilast; Diarrhoea
Introduction
Psoriasis is a frequent inflammatory skin disease, which affects about 2% of the world population. It is a chronic illness, which may have an important impact on quality of life, as described in the ESTEEM 2 trial [1]. Thus, its treatment often requires long-term use of ideally safe and effective therapeutic agents.
Among available therapeutic agents, apremilast was accepted by the European Medicines Agency (EMA) in 2015. It is the first oral phosphodiesterase 4 (PDEA4) inhibitor marketed in France (2016). It is a second line treatment for moderate to severe plaque psoriasis in case of failure or contraindication of at least two systemic treatments (cyclosporine, methotrexate and phototherapy). It is widely prescribed in France in 2019, according to the French health insurance data, 85908 apremilast medicine boxes were reimbursed (not including in hospital prescriptions).
The safety and efficacy profiles of apremilast were evaluated in the ESTEEM 1 [2] and ESTEEM 2 trials. In the ESTEEM 2 trial, the patients treated with apremilast experienced a significant benefit with a statistically significant increase in PASI 75 responses compared to placebo (reduction of 75% of Psoriasis Area and Severity Index): 28.8% vs 5.8% for placebo.
Howewer, apremilast can have several side effects, the most important of which is diarrhoea. Apremilast causes phosphodiesterase 4 inhibition and interrups the inflammatory cascade by blocking the degradation of cyclic adenosine monophosphate (cAMP). Therefore it leads to an increase in intracellular levels of cAMP in several cells types, including duodenal crypt cells. This increase activates chloride channels promoting fluid secretion into the gut lumen, leading to secretory diarrhoea. In the ESTEEM 2 trial, apremilast administration was correlated with higher incidence of diarrhoea compared to placebo (15.8% vs 5.9% for placebo).
The aim of this study is to collect the current practical interventions for the management of apremilast-induced diarrhoeas, on the basis of the literature and of a survey of the clinical practices of a few French dermatologists.
Materials and Methods
Diagnosis and grading of diarrhoea
The World Health Organisation (WHO) defines diarrhoeas as the emission of at least 3 soft or fluid stools per day. Diarrhoeas are defined as chronic when they last at least 4 weeks. The assessment of the severity is fundamental to support the choice of whether continuing, adjusting or discontinuing the treatment. Appropriate measures can be taken in order to avoid potential complications (like dehydration, acute kidney injury…): the grading is based on symptoms and the need for hospital admission. The use of Common Terminology Criteria for Adverse Events (CTCAE) is recommended (Table 1).
The World Health Organisation (WHO) defines diarrhoeas as the emission of at least 3 soft or fluid stools per day. Diarrhoeas are defined as chronic when they last at least 4 weeks. The assessment of the severity is fundamental to support the choice of whether continuing, adjusting or discontinuing the treatment. Appropriate measures can be taken in order to avoid potential complications (like dehydration, acute kidney injury…): the grading is based on symptoms and the need for hospital admission. The use of Common Terminology Criteria for Adverse Events (CTCAE) is recommended (Table 1).
| CTCAE Term | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
| Diarrhoea | Increase of < 4 stools per day over baseline ; mild increase in ostomy output compared to baseline | Increase of 4-6 stools per day over baseline ; moderate increase in ostomy output compared to baseline, limiting instrumental ADL | Increase of ? 7 stools per day over baseline ; hospitalisation indicated ; severe increase in ostomy output compared to baseline ; limiting self care ADL | Life threatening consequences ; urgent intervention indicated | Death |
ADL: activities of daily living.
CTCAE: Common Terminology Criteria for Adverse Event.
Table 1: CTCAE grade (v5.0) modified from U.S. department of health and human services.
CTCAE: Common Terminology Criteria for Adverse Event.
Table 1: CTCAE grade (v5.0) modified from U.S. department of health and human services.
Survey
An original questionnaire containing 7 multiple-choice questions and one open question was designed and internally validated by dermatologist and hepato-gastroenterologist from Le Mans hospital. The questionnaire was sent by e-mail through the ResoPso: it is a French network that gathers about 270 dermatologists active in the 5 French settings of Le Mans, Rennes, Angers, La Roche sur Yon, Paris. We collected the whole of answers from May 2020 to October 2020 and did a reminder in December 2021.
An original questionnaire containing 7 multiple-choice questions and one open question was designed and internally validated by dermatologist and hepato-gastroenterologist from Le Mans hospital. The questionnaire was sent by e-mail through the ResoPso: it is a French network that gathers about 270 dermatologists active in the 5 French settings of Le Mans, Rennes, Angers, La Roche sur Yon, Paris. We collected the whole of answers from May 2020 to October 2020 and did a reminder in December 2021.
Results
We received 176 answers. Of the respondents, 33% are private-practice dermatologists, 32.5% are hospital dermatologists and 31.8% have a combined activity.
The range of patients treated with apremilast for each of the dermatologists ranged from none to 96. 90.6% of the dermatologists had observed at least one case of apremilast-correlated diarrhoea. As for treatment choices, when patients have diarrheoas attributable to apremilast, 10.1% offer dietary and life-style interventions, 18.4% of dermatologists a symptomatic medical treatment, 65.2% a combination of the two. 6.3% of dermatologists discontinue apremilast.
Racecadotril is the most commonly medication for symptomatic treatment being used by 72.5% of the dermatologists. However, 67% of the dermatologists do not know the physiopathology of diarrhoeas caused by apremilast.
When diarrhoeas persist in spite of appropriate measures, 33.3% of them reduce the apremilast dose by half, 31.9% temporarily discontinue it and restart it at half-dose after symptom regression, while 34.7% discontinue the drug. 62.2% of dermatologists consider starting again apremilast after a period of discontinuation once diarrhoeas are over.
Discussion
Advice from the literature
Apremilast appears to be well tolerated when its posology is gradually increased (Table 2). The pathophysiology of apremilast induced diarrhea is secretory. Phosphodiesterase-4 inhibition increases in cAMP levels within bowel crypt cells. This activates enterocyte chloride channels and promotes fluid secretion into the gut lumen, which enhances bowel movement [3].
Apremilast appears to be well tolerated when its posology is gradually increased (Table 2). The pathophysiology of apremilast induced diarrhea is secretory. Phosphodiesterase-4 inhibition increases in cAMP levels within bowel crypt cells. This activates enterocyte chloride channels and promotes fluid secretion into the gut lumen, which enhances bowel movement [3].
| DAY 1 | DAY 2 | DAY 3 | DAY 4 | DAY 5 | DAY 6 | |||||
| Morning | Morning | Evening | Morning | Evening | Morning | Evening | Morning | Evening | Morning | Evening |
| 10 mg | 10 mg | 10 mg | 10 mg | 20 mg | 20 mg | 20 mg | 20 mg | 30 mg | 30 mg | 30 mg |
Table 2: Progessive rise apremilast’s posology at the initiation of the treatment (from VIDAL).
A recent observational paper of a Japanese cohort [4] of 43 patients corroborated the results of the randomized trials in a ‘real-life’ setting, and reported that the most frequent adverse event was diarrhoea. About 30% of patients reported diarrhoea, but less than 5% discontinued treatment because of this adverse event.
Quite reassuringly, these adverse events generally occurred early in the course of therapy, were rarely severe (around 0.3%), and often resolved within 4 weeks.
In a Spanish article published in 2020 [5], a multidisciplinary team of 14 experts recommend taking apremilast during meals and observing some dietetic hygiene advices (preventive measure : avoid lactose, caffeine, artificial sweeteners, eating small and frequent meals). Anti-diarrhoic agents, racecadotril seems to be the first choice, but indications drawn from this paper also mention loperamide and codeine. The autors generically suggest to reduce the dose of apremilast (30 mg per day instead of 30 mg twice a day). They also state that diarrhoeas decrease over time, and tend to disappear from the third year onwards.
Therapeutic alternatives are loperamide, which is widely used and effective in several types of diarrhoea (chemotherapy [6] or acute infectious diarrhea [7] for example). Another possible therapy is bismuth, which is occasionally used in another subtype of secretory diarrhoea, i.e. microscopic colitis [8]. To the best of our knowledge, there are no studies evaluating its efficacy in patients under apremilast.
Recently, probiotics have strongly emerged as useful agents in the management of diarrhoea. This complementary therapy, which appears to have no side effects, is often effective and therefore prescribed in order to restore a healthy and balanced intestinal microbiota with a reduction of 35% of diarrhoea (global result based on 34 studies). Nevertheless, these products are not reimbursed in France, which may limit care access [9].
Advice from the survey
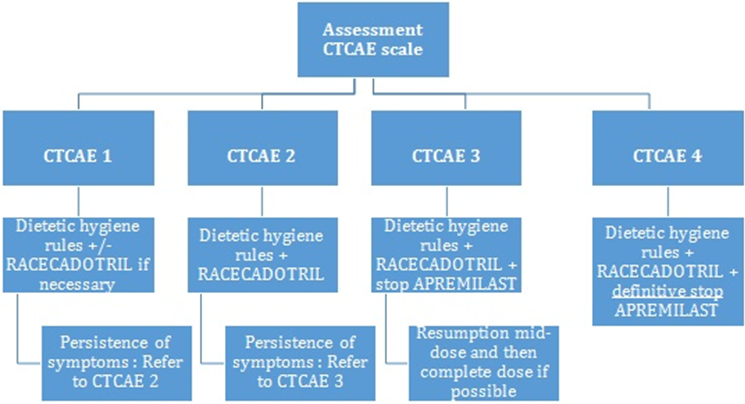
A proposed algorithm for the management of diarrhoeas under apremilast is summarized in figure 1, Management of diarrhoeas depending on CTCAE scale.
A proposed algorithm for the management of diarrhoeas under apremilast is summarized in figure 1, Management of diarrhoeas depending on CTCAE scale.
The first fundamental step is the assessment of hydration status. Adequate hydration is important in order to compensate for fluid loss. This can be achieved by increasing oral hydration or by using oral rehydration solutions (ORS) [10] which stimulate intestinal Na+ absorption, by SLC5A1 and Na+-coupled amino acid transporters.
The second step regards prevention of further episodes of diarrhoea. For this we recommend beginning with non pharmacologic interventions such as following some simple dietetic/hygiene advice: consuming small and frequent meals and paying attention to the quality of food. Some foods are to be avoided since they represent potential diarrheal triggers: milk (lactose is harder to digest because lactase is less producted in acute phase), artificial sweeteners, fibre rich foods (wholemeal bread, lentils, chickpeas…), raw fruits and vegetables, alcohol.
Eating less is not a good option though: the absence of residues in the colon leads to microbial’s pullulation, rise of fermentation, disruption of gut flora and finally, diarrhoeas.
If non-pharmacologic interventions are not sufficient, a pharmacologic treatment may be tested. Before treatment, the presence of ‘diarrhoea’s’« red flags » should be evaluated: fever, blood or mucus in the stool, recent foreign travel, antibiotic use, severe dehydration, recent hospitalization... While loperamide and diosmectite were initially recommended, racecadotril is now the first choice in secretory diarrhoeas. This drug is contraindicated in patients treated by Angiotensine-converting enzyme inhibitors (ACE-inhibitors) since it increases the risk of angioedema due to bradykinin increase.
Racecadotril is a specific inhibitor of enkephalinase, therefore prolonging the antisecretory effect of the endogenous enkephalins. Hamza et al. reported a rapid onset of action [11] and that racecadotril produced a significant decrease in stool weight within the first 24h of treatment compared to placebo (355g +/- 35 g for racecadotril group against 499 +/- 46 g for placebo group, that is to say, 28.9% of reduction).
Racecadotril is well tolerated: it does not induce constipation [12] and the patients reported less abdominal distension and abdominal pain than with loperamide [13].
The recommended dose is 100 mg three times per day, and is continued until obtaining normal stool for two days.
Finally, some cases require dose adjustment or discontinuation of apremilast. In case of failure of the previous approaches we propose to reduce by half the dose of apremilast (30 mg per day) in case of grade 2 diarrhoeas on the CTCAE scale. When diarrhoea resolves, apremilast can be increased again. In case of diarrhoeas grade 3 or 4 on the CTACE scale, stoppping treatment is probably wiser. Apremilast may be tried again in the case of diarrhoea grade 3; nevertheless, this choice seems more delicate in grade 4.
Conclusion
Apremilast is an efficient and safe therapy for psoriasis, as shown in the ESTEEM 1 and 2 trials. Diarrhoea is a frequent adverse effect, but is mostly self-limiting within 4 weeks, and does not require medical treatment in most cases. The self-limiting characteristic in apremilast induced diarrhoeas might be due to the compensatory up-regulation of other phosphodiesterases [14].
This time-limited characteristic supports a symptomatic treatment strategy. Considering the absence of guidelines regarding this topic, we recommend a step-by-step management of apremilast induced diarrhoea starting with non pharmacologic interventions. Patients not responding to these measures can be treated with racecadotril. In non-responders, dose adjustment or discontinuation, also considering the severity of the adverse effect, should be taken into consideration.
Nevertheless, these results have to be considered cautiously because we only ask, through ResoPso, dermatologists who are used to treating patients with psoriasis, even if we did this in order to get the best possible advice.
QUESTIONNAIRE
- What is your practice?*
- Self employed dermatologist
- Hospital dermatologist
- Combined activity
- How many patients receive apremilast in your cohort?*
- Do your patients have already have diarrhoeas?
- Yes
- No
- What is your first attitude in case of diarrhoea under apremilast?
- Only dietetic hygiene rules
- Only a symptomatic medical treatment
- Dietetic hygiene rules + symptomatic medical treatment
- Discontinuation of apremilast
- If you prescribe a symptomatic treatment, which one will you choose?
- Anti motility like loperamide
- Anti secretory like racecadotril
- Diosmectite
- Other
- In case of persistence of diarrhoea in spite of symptomatic treatment, what is your attitude?
- Definitive discontinuation
- Decrease the posology at 30 mg per day
- Transitory discontinuation and then restart at mid-dose (30 mg per day)
- In case of transitory discontinuation of apremilast and then restart at mid-dose, if diarrhoea is over, do you increase apremilast at full dose?
- Yes
- No
- Do you know the physiopathology of apremilast-induced diarrhoea?*
- Yes
- No
References
- Paul C, Cather J, Gooderham M, Poulin Y, Mrowietz U, Ferrandiz C, et al. (2015). Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol. Dec; 173 (6): 1387–99.
- Papp K, Reich K, Leonardi CL, Kircik L, Chimenti S, Langley RGB, et al. (2015). Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: Results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. Jul; 73(1): 37–49.
- Lambert JA, Raju SV, Tang LP, McNicholas CM, Li Y, Courville CA, et al. (2014). Cystic fibrosis transmembrane conductance regulator activation by roflumilast contributes to therapeutic benefit in chronic bronchitis. Am J Respir Cell Mol Biol. Mar; 50(3): 549–58.
- Kishimoto M, Komine M, Hioki T, Kamiya K, Sugai J, Ohtsuki M. (2018). Real-world use of apremilast for patients with psoriasis in Japan. J Dermatol. Nov; 45(11):1345–8.
- E.Daudén Tell., et al. (2021). Manejo de los efectos adversos de apremilast desde un abordaje multidisciplinar. Actas Dermo-Sifiliográficas Volume112, Issue 2, February, Pages 134-141.
- Andreyev J, Ross P, Donnellan C, Lennan E, Leonard P, Waters C, et al. (2014). Guidance on the management of diarrhoea during cancer chemotherapy. The Lancet Oncology. Sep; 15(10): e447–60.
- Douma JAJ, Smulders YM. (2015). [Loperamide for acute infectious diarrhoea]. Ned Tijdschr Geneeskd. 159:A9132.
- Kafil TS, Nguyen TM, Patton PH, MacDonald JK, Chande N, McDonald JW. (2017). Interventions for treating collagenous colitis. Cochrane Database Syst Rev. 11; 11:CD003575.
- Sazawal S, Hiremath G, Dhingra U, Malik P, Deb S, Black RE. (2006). Efficacy of probiotics in prevention of acute diarrhoea: a meta-analysis of masked, randomised, placebo-controlled trials. Lancet Infect Dis. Jun; 6(6): 374–82.
- Subramanya S, Ramakrishna BS, Binder HJ, Farthing MJ, Young GP. (2006). Evaluation of oral rehydration solution by whole-gut perfusion in rats: effect of osmolarity, sodium concentration and resistant starch. J Pediatr Gastroenterol Nutr. Nov; 43(5): 568–75.
- Hamza H, Ben Khalifa H, Baumer P, Berard H, Lecomte JM. (1999). Racecadotril versus placebo in the treatment of acute diarrhoea in adults. Aliment Pharmacol Ther. Dec; 13 Suppl 6:15–9.
- Marçais-Collado H, Uchida G, Costentin J, Schwartz JC, Lecomte JM. (1987). Naloxone-reversible antidiarrheal effects of enkephalinase inhibitors. Eur J Pharmacol. Dec 1; 144(2): 125–32.
- Roge J, Baumer P, Berard H, Schwartz JC, Lecomte JM. (1993). The enkephalinase inhibitor, acetorphan, in acute diarrhoea. A double-blind, controlled clinical trial versus loperamide. Scand J Gastroenterol. Apr; 28(4): 352–4.
- Crowley J, Thaçi D, Joly P, Peris K, Papp KA, Goncalves J, et al. (2017). Long-term safety and tolerability of apremilast in patients with psoriasis: Pooled safety analysis for ?156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol. Aug; 77(2): 310-317.e1.
Citation: Chloé Venuto, Giorgina Piccoli, Charlotte Andrianjafy, Edoardo Terzolo and Hervé Maillard. (2021). Clinical Practice Survey Regarding Management of Apremilast-Related Diarrhoeas’ in Patients with Psoriasis. Journal of Medical Research and Case Reports 3(2). DOI: 10.5281/zenodo.5814064
Copyright: © 2021 Chloé Venuto. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.