Research Article
Volume 3 Issue 1 - 2021
Changes of Reproductive Indices of the testes, Hormonal Profile and Histopathology due to T. b. brucei and T. evansi in Yankasa Rams.
1Department of Zoology, Adamawa State University, Mubi- Nigeria
2Department of Zoology, Modibbo Adama University, Yola- Nigeria
2Department of Zoology, Modibbo Adama University, Yola- Nigeria
*Corresponding Author: Asiyina Elihu, Department of Zoology, Adamawa State University, Mubi- Nigeria.
Received: September 23, 2021; Published: October 06, 2021
Abstract
Livestock production plays important roles in the provision of high-quality protein to consumers and regular income to producers but parasites especially trypanosomes have become a major threat to 3. Sustainable livestock production resulting in a decline in production. Therefore, the study was design 4.to investigate the effect of T. b. brucei and T. evansi on the changes of reproductive indices of the testis, 5. Hormonal profile and histopathology causing reproductive failures in Yankasa rams. Twelve healthy 6. and intact Yankasa rams aged between 24 and 30 months and weighed between 22 to 25 kg were 7. Purchased from Tike market Mubi. They were screened for the presence of endo and ectoparasites. 8. The rams were thereafter treated with Oxytetracycline (Tridax®) intramuscularly, at a dose of 20 mg/kg 9. Body weight and Albendazole orally, at a dose of 7.5 mg/kg body weight. The rams were sprayed against 10. Ectoparasites with Diazinon (Diaznol®, Animal Care, Nig. Ltd.). They were allowed to acclimatized for 11. Four weeks and ear-tagged for the purpose of identification in a clean fly proof house, adequately fed 12. and given water ad libitum. By the end of the four weeks acclimatization the rams were randomly grouped into three experimental groups of four rams each, based on their weights. Group A (n = 4) represent uninfected control group and group B (T.b. brucei) and C (T. evansi (n = 4 each) represent infected groups. Each infected ram received 2ml containing 2x106 trypanosomes via the jugular vein. The animals in group B had the pre-patent period of 28-49 days, which was significantly different (P<0.05) from those of group C that had not shown any prepatent parasitaemic period uptil the end of the research.
The parasites (T. b. brucei) were first observed in the peripheral circulation by day 28 post infection (p.i) with a low parasitaemic score of one plus (+). Thereafter, the rams showed progressive increase in parasitaemia, attaining a peak by day 49 p.i. with a massive parasitaemic score of three plus (+++). The hormonal profile indicates significant decrease in the testosterone level accompanied by rise in the cortisol level in infected rams compared with the normal control rams. While detached head, coil tail, bent tail and total abnormalities increased significantly (p<0.05) compared to control. Testes of the infected rams were characterized by degeneration of the seminiferous tubules, mononuclear infiltration of interstitial tissues, infiltrations by lymphocytes. It isconcluded that T. b. brucei and T. evansi infection in Yankasa rams causes severe damage to the testicular tissue and decreases the reproductive hormone levels associated with severe morphological disorders in sperms due to oxidative stress resulting from the infection.
Keywords: Reproductive indices; Testis; Hormonal profile; Yankasa rams; T.b.brucei; T.evansi
Introduction
Livestock production plays important roles in the provision of high-quality protein to consumers and regular income to producers and rearing them have been given much importance not only in developing countries but also the developed countries (Kemi, 2016). Sheep and goats constitute the world‘s largest population of livestock with an estimate of 1,173 and 1,003 million, respectively (Mazinani and Rude, 2020). In Nigeria, small ruminants (sheep and goats) are used in special ceremonies like marriages, burials, Sallah (Eid), and Christmas but livestock diseases especially trypanosomiasis remain a veritable threat to the animal production industry. Animal products are constantly under threat by diseases that affect livestock and hence reduce productivity (Amadi et al., 2015).
Trypanosomiasis have been reported to cause significant damage to reproductive aspects in animals. The reproductive harm resulted from its harmful effects on endocrine glands and gonads, which leads to hormonal perturbation either in secretion or in its concentration in the blood. Therefore, delayed puberty in young animals or disruption in semen production and its quality in adult animals were shown to occur from infection by trypanosoma sp (Amin et al., 2020). The disease is noted for its economic effects in the form of reduced milk production, abortion, infertility, sterility, reduced parity (Agada et al., 2018).
The pathogenesis of trypanosome-induced reproductive dysfunction resulting in reduction of circulating Luteinizing Hormone, testosterone in males and Follicle Stimulating Hormone in female. These together with direct testicular lesions affect spermatogenesis resulting to poor semen quality and infertility in males while ovarian lesions along with endometritis cause irregular estrous cycle, infertility, feotal deaths and abortion in females (Raheem, 2014). This had been collaborated in the findings of Ogbaje et al. (2017) in T. b. brucei and T. congolense infected Yankasa sheeps.
The presence of T. b. brucei in the genital tract and the brain, in addition to severe lesions resulting in the degeneration of testes which involved the Leydig cells, basement membrane, Sertoli and germ cells, with resultant loss of libido was reported in Yankasa rams (Wada et al., 2016).
Clinically, the effects of trypanosomosis on these animals ranges from anaemia, immunosuppression, depression with inability to rise, pyrexia directly associated with parasitaemia, paleness of mucous membrane, rapid pulse beat, retarded growth, roughness of hair coats, enlargement of peripheral lymph nodes, low milk production, low meat quality, weight loss and reproductive disorders, including degeneration of the hypothalamus, pituitary glands and gonads with consequent disruptions in the secretions and plasma concentrations of the hormones necessary for normal reproductive processes in both sexe (Silva et al.,2016).
This study describes that T. b. brucei and T. evansi infection in Yankasa rams causes marked testicular tissue damage and a decrease in the levels of hormones of the reproductive system such as testosterone and severe morphological disorders in sperms due to the increase in the levels of cortisol. All these findings indicate the incidence of complete reproductive failure, which supports the idea that this parasite can contribute to infertility in Yankasa rams. Therefore, this study aimed to investigate the changes of reproductive indices of the testis, hormonal profile and histopathology due to T. b. brucei and T. evansi infection.
Materials and Methods
Ethical statement
The steps of the current study were carried out according to the ethical guidelines governing the use of laboratory animals in research with permission from the Animal Welfare Unit of the Department of Animal Production Teaching and Research farm, Adamawa State University Mubi, Nigeria.
The steps of the current study were carried out according to the ethical guidelines governing the use of laboratory animals in research with permission from the Animal Welfare Unit of the Department of Animal Production Teaching and Research farm, Adamawa State University Mubi, Nigeria.
Experimental Animals and grouping
Twelve apparently healthy and intact Yankasa rams aged between 24 and 30 months and weighed between 22 to 25 kg were purchased from local market around Mubi. Their age was estimated using the pattern of eruption of their dentition, while breeding history was obtained from the sellers where possible. This study was carried out in the breeding season (January to March). By the end of the four weeks acclimatization period rams were randomly grouped into three experimental groups (A, B and C) of four rams each, based on their weights. The rams in groups B, C were experimentally infected with T.b. brucei, T. evansi respectively while those in group A served as the uninfected control.
Twelve apparently healthy and intact Yankasa rams aged between 24 and 30 months and weighed between 22 to 25 kg were purchased from local market around Mubi. Their age was estimated using the pattern of eruption of their dentition, while breeding history was obtained from the sellers where possible. This study was carried out in the breeding season (January to March). By the end of the four weeks acclimatization period rams were randomly grouped into three experimental groups (A, B and C) of four rams each, based on their weights. The rams in groups B, C were experimentally infected with T.b. brucei, T. evansi respectively while those in group A served as the uninfected control.
Collection of samples and diagnosis of trypanosome infection
Blood was collected from rams by jugular venipuncture. Blood smears were observed daily for 90 days. Microscopic examination was done at 40× and focused on the number of the parasite per field. Level of parasitaemia was determined by using haematocrit centrifugation technique (HCT) as adopted by Wada et al. (2016). The procedure involved filling two heparinised micro-capillary tubes (75x1.5mm) to approximately two-third of their volumes with each of the infected blood. The tubes were sealed with a sealant and thereafter, placed in a micro-haematocrit centrifuge in an opposite direction to be balanced, while the sealed ends was allowed to face outwards. It was spinned for 3 minutes at 1500 revolutions per minute (rpm). The spun capillary tubes were thereafter placed on a glass slide and oil immersion was applied on the buffy coat area and viewed under the objective lens (X40) to determine parasitaemic scores as described by (OIE, 2018).
1. + = less than 10 trypanosomes in buffy coat or plasma layer seen per field.
2. ++ = 10 - 20 trypanosomes in buffy coat or plasma layer seen per field.
3. +++ = Numerous (20 – 30) trypanosomes in buffy coat or plasma layer seen per field.
4. ++++ = massive (30 – 40) trypanosomes in buffy coat or plasma layer seen per field.
Blood was collected from rams by jugular venipuncture. Blood smears were observed daily for 90 days. Microscopic examination was done at 40× and focused on the number of the parasite per field. Level of parasitaemia was determined by using haematocrit centrifugation technique (HCT) as adopted by Wada et al. (2016). The procedure involved filling two heparinised micro-capillary tubes (75x1.5mm) to approximately two-third of their volumes with each of the infected blood. The tubes were sealed with a sealant and thereafter, placed in a micro-haematocrit centrifuge in an opposite direction to be balanced, while the sealed ends was allowed to face outwards. It was spinned for 3 minutes at 1500 revolutions per minute (rpm). The spun capillary tubes were thereafter placed on a glass slide and oil immersion was applied on the buffy coat area and viewed under the objective lens (X40) to determine parasitaemic scores as described by (OIE, 2018).
1. + = less than 10 trypanosomes in buffy coat or plasma layer seen per field.
2. ++ = 10 - 20 trypanosomes in buffy coat or plasma layer seen per field.
3. +++ = Numerous (20 – 30) trypanosomes in buffy coat or plasma layer seen per field.
4. ++++ = massive (30 – 40) trypanosomes in buffy coat or plasma layer seen per field.
Reproductive Parameters
Reproductive parameters such as sperm count or concentration, motility, viability (life/dead %), sperm morphology, abnormalities and serum testosterone and cortisol was measured.
Reproductive parameters such as sperm count or concentration, motility, viability (life/dead %), sperm morphology, abnormalities and serum testosterone and cortisol was measured.
Semen collection and evaluation.
Semen collection was done weekly between 9am and 10am, by electro stimulation with the help of a portable battery-powered electro-ejaculatory mini tube (Lane Ram Ejaculator, model C27113) for small ruminants. The rams were adequately restrained, the prepuce was washed and dried using cotton wool soaked in diluted chloroxylenol (0.002%; DettolR) to remove dirts and debris. The probe of the electro-ejaculator was lubricated using petroleum jelly and inserted into the animal‘s rectum and switched on, this result in erection and subsequently, ejaculation. Semen begins to flow once the animal has achieved excitation by the stimulatory action of the electroejaculatory device. The impulses consist of applying the stimulus at an interval of 5 seconds, with 5seconds’ break. The ejaculates were collected into pre-warmed, sterile and graduated transparent collection tube, labelled and kept in a water bath at a temperature range of 35-37ºC. This was done to prevent temperature changes which may affect the quality of semen before analysis.
Semen collection was done weekly between 9am and 10am, by electro stimulation with the help of a portable battery-powered electro-ejaculatory mini tube (Lane Ram Ejaculator, model C27113) for small ruminants. The rams were adequately restrained, the prepuce was washed and dried using cotton wool soaked in diluted chloroxylenol (0.002%; DettolR) to remove dirts and debris. The probe of the electro-ejaculator was lubricated using petroleum jelly and inserted into the animal‘s rectum and switched on, this result in erection and subsequently, ejaculation. Semen begins to flow once the animal has achieved excitation by the stimulatory action of the electroejaculatory device. The impulses consist of applying the stimulus at an interval of 5 seconds, with 5seconds’ break. The ejaculates were collected into pre-warmed, sterile and graduated transparent collection tube, labelled and kept in a water bath at a temperature range of 35-37ºC. This was done to prevent temperature changes which may affect the quality of semen before analysis.
Gross sperm motility
This was determined according to the method adopted by Wada et al. (2016) by placing a drop of raw undiluted semen on a pre-warmed slide then cover-slipped and viewed using a field microscope at X40 magnification. The results were scored objectively using the scoring pattern in percentages as presented below:
90-100% Excellent, continuous progressive motility
80-89% Very good, continuous progressive motility
70-79% Good, continuous progressive motility
60-69% Shift continuous progressive motility
50-59% Very active-none-progressive motion
40-49% Shift none-progressive motion
10-19% No motion
This was determined according to the method adopted by Wada et al. (2016) by placing a drop of raw undiluted semen on a pre-warmed slide then cover-slipped and viewed using a field microscope at X40 magnification. The results were scored objectively using the scoring pattern in percentages as presented below:
90-100% Excellent, continuous progressive motility
80-89% Very good, continuous progressive motility
70-79% Good, continuous progressive motility
60-69% Shift continuous progressive motility
50-59% Very active-none-progressive motion
40-49% Shift none-progressive motion
10-19% No motion
Sperm concentration
Sperm concentration was evaluated by visual count under the microscope using improved Neubauer Haemocytometer. The sperm cells were immobilized using a 1% formaldehyde solution prior to counting. The raw semen was mixed thoroughly and filled into the unopette capillary tube with a dilution ratio of 1:10. The diluted semen was thereafter transferred unto the haemocytometer chamber and counted under the microscope. The number of sperm cells counted using the haemocytometer multiplied by a million (106) was the concentration per ml of the raw semen.
Sperm concentration was evaluated by visual count under the microscope using improved Neubauer Haemocytometer. The sperm cells were immobilized using a 1% formaldehyde solution prior to counting. The raw semen was mixed thoroughly and filled into the unopette capillary tube with a dilution ratio of 1:10. The diluted semen was thereafter transferred unto the haemocytometer chamber and counted under the microscope. The number of sperm cells counted using the haemocytometer multiplied by a million (106) was the concentration per ml of the raw semen.
Percentage live- dead spermatozoa
The percentage live sperm and spermatozoa morphological abnormalities were determined using Eosin-Nigrosin stain technique, applied on a glass slide. One drop of raw semen was added to one drop of the stain, thereafter it was mixed thoroughly and a fresh smear was made from it. The slide was then examined under a light binocular microscope at X40 magnification. A minimum of 100 spermatozoa was counted and the percentage of each estimated. The live-dead staining principle was based upon the observation that Eosin-B penetrate the dead sperms (thereby making them appear pink), while the viable sperm cells repelled the stain and appeared unstained (white).
The percentage live sperm and spermatozoa morphological abnormalities were determined using Eosin-Nigrosin stain technique, applied on a glass slide. One drop of raw semen was added to one drop of the stain, thereafter it was mixed thoroughly and a fresh smear was made from it. The slide was then examined under a light binocular microscope at X40 magnification. A minimum of 100 spermatozoa was counted and the percentage of each estimated. The live-dead staining principle was based upon the observation that Eosin-B penetrate the dead sperms (thereby making them appear pink), while the viable sperm cells repelled the stain and appeared unstained (white).
Spermatozoa abnormal morphology
Morphological abnormalities of the spermatozoa that were examined include incidence of Detached Head (DH), Bent Tail (BT) and incidence of Coiled Tail (CT). These abnormalities were determined by making a thin smear of the semen sample on clean grease-free glass slide and fixed with buffered formal saline. Semen samples stained with eosin-nigrosin were used to determine the morphological abnormalities of the sperm head. A sperm cell was counted per slide using light microscopy at X40 magnification and was classified and calculated as adopted by Wada et al., (2016).
Morphological abnormalities of the spermatozoa that were examined include incidence of Detached Head (DH), Bent Tail (BT) and incidence of Coiled Tail (CT). These abnormalities were determined by making a thin smear of the semen sample on clean grease-free glass slide and fixed with buffered formal saline. Semen samples stained with eosin-nigrosin were used to determine the morphological abnormalities of the sperm head. A sperm cell was counted per slide using light microscopy at X40 magnification and was classified and calculated as adopted by Wada et al., (2016).
Hormonal analysis
Serum Testosterone and Cortisol analysis
Blood was obtained through puncture into vaccutainer tubes. It was then centrifuged at 1500rpm for 5 min and serum obtained was stored at -20°C until laboratory analysis was carried out. Serum testosterone was determined using testosterone enzyme immunosorbent assay (ELISA) kit (Diagnostic Automation/Cortes Diagnostic Inc. Immuno Diagnostics CA, Woodland Hills, Califonia) according to the manufacturer’s instructions. Cortisol level was estimated using AccuDiagTM Sheep cortisol ELISA kits.
Serum Testosterone and Cortisol analysis
Blood was obtained through puncture into vaccutainer tubes. It was then centrifuged at 1500rpm for 5 min and serum obtained was stored at -20°C until laboratory analysis was carried out. Serum testosterone was determined using testosterone enzyme immunosorbent assay (ELISA) kit (Diagnostic Automation/Cortes Diagnostic Inc. Immuno Diagnostics CA, Woodland Hills, Califonia) according to the manufacturer’s instructions. Cortisol level was estimated using AccuDiagTM Sheep cortisol ELISA kits.
Histopathology
For histopathological evaluation, samples from testes were collected. Two slides were made for each sample, which was stained by Haematoxylin and Eosin (H & E) method to determine the degree of its degeneration.
For histopathological evaluation, samples from testes were collected. Two slides were made for each sample, which was stained by Haematoxylin and Eosin (H & E) method to determine the degree of its degeneration.
Statistical analysis
Statistical evaluation of the results was carried out using the Statistical Analysis for Sciences (SAS), version 2002.The values of P < 0.05 was considered statistically significant.
Statistical evaluation of the results was carried out using the Statistical Analysis for Sciences (SAS), version 2002.The values of P < 0.05 was considered statistically significant.
Results
The results of this study showed a comparison between the infected groups and the control group in some parameters.
Progression of parasitaemia
Following inoculation with the trypanosome species, some of the infected groups of rams developed parasitaemia at varying pre-patent periods as shown in Figure 1. The animals in group B (T. b. brucei- infected rams) had the pre-patent period of 28-49 days, which was different from those of group C (T. evansi infected rams). The parasites (T. b. brucei) were first observed in the peripheral circulation by day 28 post infection (p.i.) in two of the infected rams (R82 and R100) in group B, with a low parasitaemic score of one (1) plus (+). Thereafter, there was an observed progressive increase in parasitaemia, attaining a peak by day 49 p.i. (Figure 1) with a massive parasitaemic score of three plus (+++). The parasites disappeared from the peripheral blood of the rams by 56 days p.i. up to the end of the experiment. Among all the infected groups, parasitaemia, with T. b brucei infected rams had the highest level of parasitaemic score. All the rams in the uninfected control group (A) remained aparasitaemic throughout the experimental period.
Following inoculation with the trypanosome species, some of the infected groups of rams developed parasitaemia at varying pre-patent periods as shown in Figure 1. The animals in group B (T. b. brucei- infected rams) had the pre-patent period of 28-49 days, which was different from those of group C (T. evansi infected rams). The parasites (T. b. brucei) were first observed in the peripheral circulation by day 28 post infection (p.i.) in two of the infected rams (R82 and R100) in group B, with a low parasitaemic score of one (1) plus (+). Thereafter, there was an observed progressive increase in parasitaemia, attaining a peak by day 49 p.i. (Figure 1) with a massive parasitaemic score of three plus (+++). The parasites disappeared from the peripheral blood of the rams by 56 days p.i. up to the end of the experiment. Among all the infected groups, parasitaemia, with T. b brucei infected rams had the highest level of parasitaemic score. All the rams in the uninfected control group (A) remained aparasitaemic throughout the experimental period.
General clinical observations:
For the rams that served as control, there were no significant clinical changes associated with the group throughout the study period. The observed clinical signs among the rams in the infected Groups B, and C were similar and include: pale ocular membrane, reduced feed intake, and reduced body weight gain, rough hair coat, scrotal oedema, scrotal degeneration, and poor semen output, loss of libido, drowsiness and death. Rectal temperature was significantly higher (p<0.05) in all the groups (Table 1).
For the rams that served as control, there were no significant clinical changes associated with the group throughout the study period. The observed clinical signs among the rams in the infected Groups B, and C were similar and include: pale ocular membrane, reduced feed intake, and reduced body weight gain, rough hair coat, scrotal oedema, scrotal degeneration, and poor semen output, loss of libido, drowsiness and death. Rectal temperature was significantly higher (p<0.05) in all the groups (Table 1).
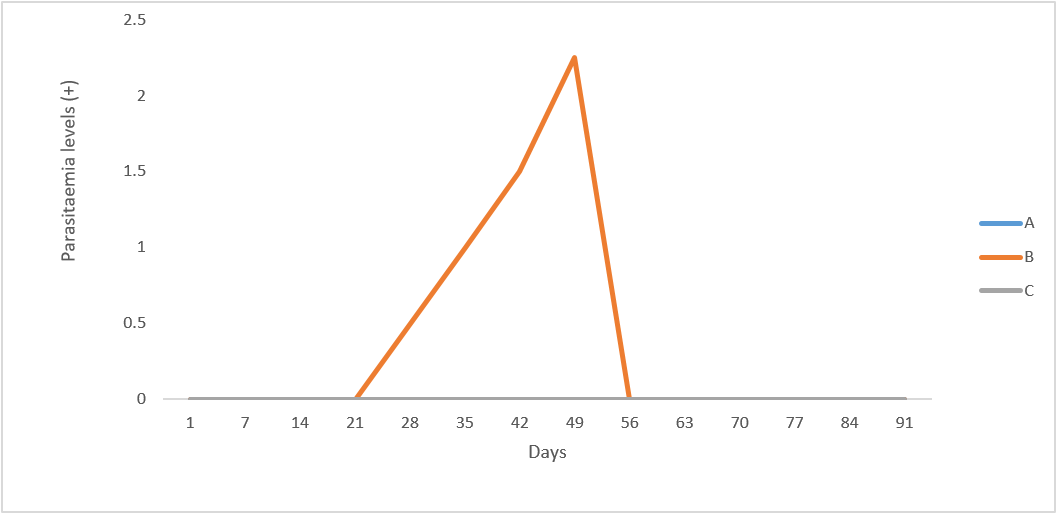
Figure 1: Mean parasitaemic scores of uninfected control Yankasa rams (A) and rams experimentally infected with T. b. brucei (B) and T. evansi (C).
| Control Group Infected Groups (n = 8) | |||
| Parameters | A (n = 4) | B (n= 4) T.b. brucei | C (n = 4) T. evansi |
| Rectal temperature (°C) | 38.84a ± 0.06 | 38.83a ± 0.08 | 38.63a ± 0.10 |
| Sperm Count (x 106/ml) | 697.14a ± 56.91 | 657.78a ± 44.00 | 642.76a ± 51.81 |
| Motility (%) | 39.93c ± 5.18 | 41.83a ± 10.21 | 43.47a ± 5.19 |
| Live sperm (%) | 40.14a ± 5.13 | 43.60b ± 4.21 | 44.50b ± 5.26 |
| Abnormalities (%) | 0.18c ± 0.08 | 3.53a ± 0.42 | 2.16b ± 0.41 |
Table 1: Estimates of clinical and seminal parameters in infected and control group (means ± standard errors).
Values in the same row having the same superscripts does not differ significantly (p>0.05)
Values in the same row having the same superscripts does not differ significantly (p>0.05)
Sperm morphology
Examination of sperm morphology revealed the presence of an increase in the percentage of abnormalities (p < 0.05) in the infected animals. Significant changes were observed in the head (detached head), bent tail, and coil tail of the sperms in infected Yankasa rams compared to the uninfected rams (Table 2).
Examination of sperm morphology revealed the presence of an increase in the percentage of abnormalities (p < 0.05) in the infected animals. Significant changes were observed in the head (detached head), bent tail, and coil tail of the sperms in infected Yankasa rams compared to the uninfected rams (Table 2).
| Control Group Infected Groups (n = 8) | |||
| Parameters | A (n = 4) | B (n= 4) T.b. brucei | C (n = 4) T. evansi |
| Detached heads (%) | 0.06c ± 0.04 | 1.93a ± 1.93 | 1.03b ± 0.31 |
| Bent tail (%) | 0.06c ± 0.04 | 1.16a ± 1.18 | 0.68b ± 0.14 |
| Coil tail (%) | 0.06b ± 0.06 | 0.44a ± 0.09 | 0.45a ± 0.13 |
Table 2: Morphological analysis of sperm in infected and control groups.
Values in the same row having different superscripts differ significantly (p<0.05).
Values in the same row having different superscripts differ significantly (p<0.05).
Hormonal profile
Examination of the hormonal profile (testosterone and cortisol) of the infected rams revealed that these animals suffered from significant decrease in testosterone and increased cortisol levels (p < 0.05) when compared to the control group (Figure 2).
Examination of the hormonal profile (testosterone and cortisol) of the infected rams revealed that these animals suffered from significant decrease in testosterone and increased cortisol levels (p < 0.05) when compared to the control group (Figure 2).
Histopathology
The testes of the control Yankasa rams (A) showed normal tissue architecture with normal active semiferous tubules containing proliferating spermatogenic cell layers and supportive sertoli cells. There was matured spermatid within the lumen of the seminiferous tubule (Plate 1a) but T. b. brucei-infected groups (B)), there was severe atrophic and distorted seminiferous tubule containing degenerating spermatogenic cells and sertoli cells (Plate 1b), while T. evansi infected Yankasa rams (C) showed moderate degeneration (Plate 1c)
The testes of the control Yankasa rams (A) showed normal tissue architecture with normal active semiferous tubules containing proliferating spermatogenic cell layers and supportive sertoli cells. There was matured spermatid within the lumen of the seminiferous tubule (Plate 1a) but T. b. brucei-infected groups (B)), there was severe atrophic and distorted seminiferous tubule containing degenerating spermatogenic cells and sertoli cells (Plate 1b), while T. evansi infected Yankasa rams (C) showed moderate degeneration (Plate 1c)
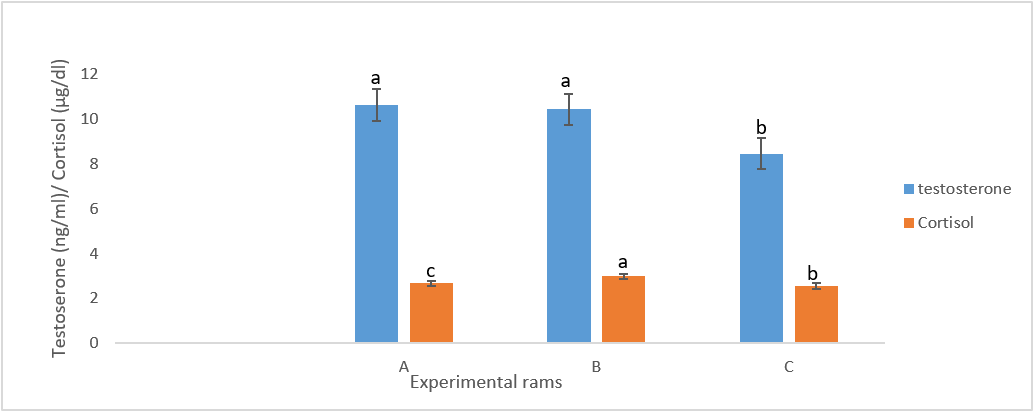
Figure 2: Hormonal parameters of infected groups compared to the control group. Bars with same colour having different superscripts differ significantly (p<0.05).
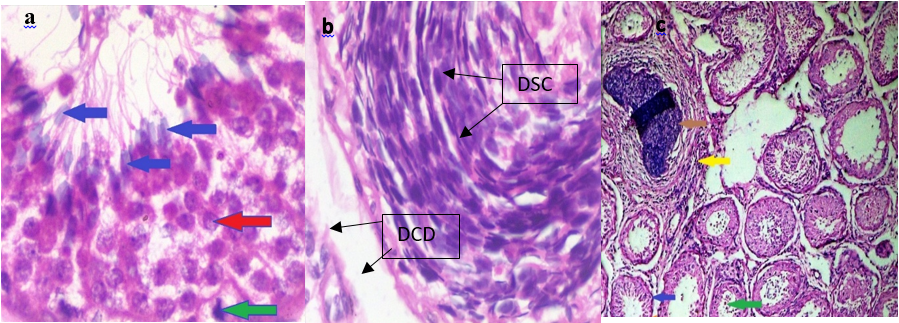
Plate 1: Histopathological examination of testes of control group (A) shows active semiferous tubules containing spermatogenic cells and sertoli cells (red arrow), sperm cells (blue arrow) and immatured cells (green arrow) (Plate 1a). T. b. brucei infected group (B) shows severe testicular degeneration of spermatogenic cells (DSC) and extensive degenerative cells desquamation (DCD) within the lumen of the tubules (Plate 1b) while T. evansi infected group (C) shows degenerative spermatogenic and sertoli cells (blue arrow), degenerative cells desquamation within the lumen of the tubules (green arrow), focal area of calcification (brown arrow), interstitial cells infiltration with mononuclear cells (yellow arrow) Plate 1c. (H & E stains x 400).
Discussion
In the present study, the clinical signs and gross pathological lesions encountered in the infected animals include: pale ocular membrane, reduced feed intake, reduced body weight gain, rough hair coat, scrotal oedema, scrotal degeneration, and poor semen output, loss of libido, incoordination, nervous manifestations like convulsion and death. This finding is congruent with the findings of Ogundele et al. (2016). There was rise in temperature of infected rams following inoculation which is in line with the report of Ogundele et al. (2016) who reported rise in temperature of T. evansi infection in goat.
The prepatent period of 28 to 49 days for jugular vein inoculation of T. b. brucei (Emodike strain) and non-prepatent period in T. evansi observed in this study is in contrast with prepatent period of 5 to 6 days intraperitoneal inoculation of T.b. brucei and T. congolense reported in WAD sheeps by Anyogu et al. (2020) and mean prepatent period of 3.8 days for T. b. b. brucei and 6.5 days for T. congolense in mice by Ndungu et al. (2019), 7-11 days post infection in T. congolense infected Yankassa rams by Okubanjo et al(2015) and 3-4 days in Swiss white mouse model infected with T. b. rhodesiense.
In the current study examination of the semen showed that infected group was significantly lower in semen concentration compared to the uninfected control animals. There was high significant decrease in semen concentration in millions per mL of all the infected group when compared to the control Group.
This report agrees with the work of Wada et al. (2016) who reported significant decrease in semen concentration in millions per ml of all the infected Groups of Yankasa rams experimentally infected with T. b. brucei and T. evansi and Ogundele et al. (2016) who also reported decrease in sperm concentration in Yankasa rams infected T. evansi. Among all the infected groups, semen concentration was lowest in T. evansi.
Study on the sperm morphology revealed the presence of an increase in the percentage of abnormalities (p < 0.05) in the infected animals. The significant changes were observed in the head (detached head) and tail (coil tail, bent tail) of the sperms in infected rams compared to the uninfected. This study agrees with the report of Amin et al. (2020) who reported spermatozoa abnormalities of the head and tail of dromedary bulls infected with T. evansi. The highest mean percent spermatozoa abnormalities were observed in rams infected with T. b. brucei and the lowest observed in the control group also agrees with the report of Wada et al. (2016). There was a significant (P<0.05) increase in the mean detached heads of the infected rams compared to the control. This result is in agreement with the report of Okubanjo et al. (2015) in T. congolense infected Yankasa rams and Ubah et al. (2017) who reported same in rams following cypermethrin treatment.
Examination of the hormonal testosterone profile of the infected Yankassa rams revealed that these animals suffered from a significant decrease in the levels of testosterone when compared to the control group. These results are similar to the report of Amin et al. (2020) who showed that testosterone level decreased in dromedary bulls infected with T. evansi and also Okubanjo et al. (2015) confirmed that the experimentally infected male rat with T. congolense suffered from a reduction of testosterone, luteinizing hormone (LH) and follicle stimulating hormone (FSH) levels. The significant differences observed among the treatment group in testosterone production are in agreement with the report of Egu, (2017) who recorded significant difference among treatment group in serum testosterone production in Ouda rams. This result is also in agreement with the report of Maksimovi? et al. (2016) who reported same in Meat Institute Sheep (MIS) Serbia, Belgrade.
In this study, serum of infected Yankasa rams contains high levels of cortisol which is in consonance with the report of Amin et al. (2020) who also recorded high level of cortisol in dromedary camels infected with T. evansi and also this result is in agreement with the report of Faccio et al. (2014) who reported increased cortisol in male rats infected with T. evansi. This result is also in agreement with the report of Alomar et al. (2016) who reported high level of cortisol in an experiment on testosterone and cortisol patterns and the effects of electro-ejaculation and copulation in Awassi rams.
Histopathology of the testis from infected Yankasa rams in the current study showed degenerative changes of the seminiferous tubules characterized by vacuolation with dead spermatocytes in T. evansi. The same result was recorded in dromedary camels (Amin et al., 2020). Also, similar results were detected in the experiment of rams infected with T. b. brucei and T. evansi (Wada et al., 2016). Furthermore; necrosis and inflammation, calcified cells and monocytes infiltrations, interstitial cells with white blood cells, myofibrin were recorded in this study which was also in agreement with the report of Amin et al., (2020), Ogundele et al., (2016) and Kothari et al., (2017) who reported on the effect of T. vivax on reproductive organs of sheep and goats.
Conclusion
It is concluded that T. b. brucei and T. evansi infection in Yankasa rams causes severe damage to the testicular tissue and a decrease in the reproductive hormone levels associated with severe morphological disorders in sperms due to oxidative stress resulted from the infection. All these findings indicate tha T. b. brucei infection induced complete reproductive failure, leading to infertility in male camels, either directly or indirectly. Trypanosomiasis due to T. b. brucei and T. evansi infections resulted in marked increase in spermatozoa morphological abnormalities of Yankasa rams which may render the rams infertile and unfit for breeding if untreated.
Acknowledgement
The author is grateful to Mr. Joel Isa, and all staff of the Department of Animal Production Teaching and Research Farm Adamawa State University, Mubi- Nigeria for their technical assistance. The pathologist, Mr Alex Akaosi and Mr Ferdinand Medugu of the Federal Medical Centre Yola and the Management of Adamawa State University, Mubi-Nigeria, is also appreciated for granting me a study fellowship (Ph.D).
The author is grateful to Mr. Joel Isa, and all staff of the Department of Animal Production Teaching and Research Farm Adamawa State University, Mubi- Nigeria for their technical assistance. The pathologist, Mr Alex Akaosi and Mr Ferdinand Medugu of the Federal Medical Centre Yola and the Management of Adamawa State University, Mubi-Nigeria, is also appreciated for granting me a study fellowship (Ph.D).
References
- Agada C.A, Ogugua A.J and Anzaku E.J (2018). Occurrence of brucellosis in small ruminants slaughtered in Lafia central abattoir, Nasarawa State, Nigeria. Sokoto Journal of Veterinary Sciences, 16(1): 16 - 23.
- Alomar, M; Soukouti, A; Alzoabi, M.A and Zarkawi, M (2016). Testosterone and cortisol patterns and the effects of electro-ejaculation and copulation in Awassi rams. Archives Animal Breeding. 59: 139–144.
- Amadi, A.N.C, Okore, I.B, Amajuonwu, A. (2015). Haematological studies on West African Dwarf (WAD) bucks experimentally infected with Trypanosoma vivax and Trypanosoma brucei and response to treatment with diaminazene aceturate. Global Journal of Medical Research: G Veterinary Science and Veterinary Medicine; 15: 2249-4618.
- Amin, Y.A, Noseer E.A, Fouad,S.S, Ali R.A, Mahmoud H.Y.A.H. (2020).Changes of reproductive indice of the testis due to Trypanosoma evansi infection in dromedary bulls (Camelus dromedarius) Semen picture hormonalprofile, histopathology, oxidative parameters, and hematobiochemical profile. Journal Advance Vetinary . Animal Research 2020; 7(3): 537–545.
- Anyogu, D.C, Shoyinka, V.O, Ihediola, J.I (2020). Some Biochemical Pertubations May Modify the Understanding of Trypanotolerance in West African Dwarf sheep infected with Trypanosoma brucei brucei and Trypanosoma congolense. Clinical Pathology, 13: 1-6.
- Egu, U.N and Okonkwo, J.C (2017). Effect Of Gonadotrophin (Diclair®) On Semen Characteristics, Hormonal Profile And Biochemical Constituents Of The Seminal Plasma Of Mature Balami Rams. Banat Journal of Biotechnology, VIII (15): 90-97.
- Faccio L, Silva A.S, Tonin A.A, Oberherri L, Gressler L.T, Oliveie C.B et al. (2014). Relationship between testicular lesions and hormones levels in male rats infected with Trypanosoma evansi. Annals of Brazilian Academy of Sciences. 86: 1537-46.
- Kem,i A.O. (2016). Economic Impact of Livestock Production on the Society: A Case Study of Ikare Akoko Ondo State. IOSR Journal of Agriculture and Veterinary Science (IOSR-JAVS) 9, (12): 77-80.
- Kothari, R., Waghmare, S. and Choundhari, A.R. (2017). Role of Ascorbic Acid in Male Fertility and its Relation with Free Testosterone. International Journal of Comtemporary Medical Research. 4(6):77-83.
- Maksimovic N., Hristo, S., Stankovic, B, Mekev, C, Ruzio-Muslic, D and Caro-Petrovic, V. (2016). Investigation of serum testosterone level, scrotal circumference, body mass, semen characteristics, and their correlations in developing MIS lambs. Turkish Journal of Veterinary Animal Science. 40: 53-59.
- Mazinani, M. and Rude, B. (2020). Population, World Production and Quality of Sheep and Goat Products. American Journal of Animal and Veterinary Sciences, 15(4): 291-299.
- Ndungu, K. Thungu, D. Wamwiri, F et al., (2019). Route of inoculation influences Trypanosoma congolense and Trypanosoma brucei brucei virulence in Swiss white mice. Plos ONE.14: E0218441.
- Office of the International Epizootic (OIE) (2018). Trypanosoma evansi infection (Surra).OIE Terrestrial Manual. pp. 1-15.
- Ogbaje, C.I, Lawal, I.A. and Ajanusi, O.J. (2017). Comparative clinical presentation of some organs in experimentally infected yankasa sheep with T.vivax, T.congolence from Nigeria. Turk Journal of Vetinary Animal Science 41: 604-612.
- Ogundele, F.A., Okubanjo, O.O., Ajanusi, O.J. and Fadason, S.T. (2016). Effect of experimental Trypanosoma evansi infection on Haematological parameters and semen characteristic of Yankasa rams. Theriogenology, 86: 667-673.
- Okubanjo O.O., Sekoni V. O., Ajanusi O.J., Adeyeye A.A(2015). Effects of experimental Trypanosoma congolense infection on sperm morphology in Yankasa rams. Mac Veterinary Review; 38 (2): 203-208.
- Raheem K.A (2014). A Review of Trypanosomosis-Induced Reproductive Dysfunctions in Male Animals. Agrosearch. Volume 14(1): 30-38.
- Silva, J. F., Capettini, L.S.A., da Silva, J.F.P., Sales- Junior P et al., (2016). Mechanisms of vascular dysfunction in acute phase of Trypanosoma cruzi infection in mice. Vascular Pharmacology 82: 73-81.
- Ubah, S.A. , Ogwu, D. , Rekwot, P.I. and Rwuaan, J.S. (2017). Specific sperm abnormalities observed in rams ( ovis aries) following Cypermethrin treatment. Journal of Cell and Animal Biology, 11(1): 1-6.
- Wada, Y.A., Oniye, S.J, Rekwot P.I and Okubanjo, O.O(2016a). Testicular pathology, gonadal and epididymal sperm reserves of Yankasa rams infected with experimental T. b.brucei and T. evansi. Veterinary World, 9(7): 759-765.
Citation: Elihu A and Naphtali RS. (2021). Changes of Reproductive Indices of the testes, Hormonal Profile and Histopathology due to T. b. brucei and T. evansi in Yankasa Rams. Journal of Medical Research and Case Reports 3(1).
Copyright: © 2021 Asiyina Elihu. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
