Case Report
Volume 2 Issue 2 - 2020
A Case of Preschoolar Girl Presented with Progressive Huge Distended Abdominal Diagnosed to be Huge Ovarian Cyst Managed by Explorative Laparotomy: Histopathology Revealed Endodermal Sinus (yolk sac) Ovarian Tumour.
1Department of Obstetrics and Gynaecology, the University of Dodoma, College of Health Sciences P.O BOX 395 Dodoma, Tanzania
2Department of Obstetrics & Gynaecology, Catholic University of Health & Allied Sciences P.O BOX 1464 Mwanza, Tanzania
3Department of Obstetrics & Gynaecology, Catholic University of Health & Allied Sciences P.O BOX 1464 Mwanza, Tanzania
4Department of Obstetrics & Gynaecology, Catholic University of Health & Allied Sciences P.O BOX 1464 Mwanza, Tanzania
2Department of Obstetrics & Gynaecology, Catholic University of Health & Allied Sciences P.O BOX 1464 Mwanza, Tanzania
3Department of Obstetrics & Gynaecology, Catholic University of Health & Allied Sciences P.O BOX 1464 Mwanza, Tanzania
4Department of Obstetrics & Gynaecology, Catholic University of Health & Allied Sciences P.O BOX 1464 Mwanza, Tanzania
*Corresponding Author: Athanase Lilungulu, Department of Obstetrics and Gynaecology, the University of Dodoma, College of Health Sciences P.O BOX 395 Dodoma, Tanzania.
Received: July 04, 2020; Published: July 20, 2020
Abstract
Background: Endodermal sinus (yolk sac) is a very rare tumor typically affects girls and young women. They tend to grow and spread rapidly but are usually very sensitive to chemotherapy. The presentation of an adnexal mass in preschoolar girls is a concerning event for the patient and the family. Patients are most likely to present with abdominal pain or a palpable mass. The diagnostic imaging of choice is pelvic ultrasonography. The majority of these lesions are benign ovarian cysts. These cysts should be followed with ultrasound, as many will spontaneously regress. Further evaluation with laboratory tests may also be warranted, which will aid in diagnosis and treatment.
Ovarian cysts that are failed to resolve, severely symptomatic, or concerned with malignancy require surgical intervention. Laparoscopy has shown to be beneficial in the adolescent population and should be the procedure of choice. In addition, ovarian conservation is the ideal treatment in order to continue normal pubertal development and preserve reproductive health.
Introduction
Adnexal masses are uncommon occurrences in the pre scholar and adolescent population. However, when they occur, they are anxiety-provoking for both the patient and her family. Many practitioners are unfamiliar with the proper management of these adnexal masses and are quick to proceed with surgical intervention that is often unnecessary (1). The estimated incidence of adnexal masses in the preschoolar and adolescent population is approximately 2.6 per 100,000 girls younger than 18 years of age (2). However, an estimated number of the overall identified population with adnexia mass in preschoolar and adolescent very few of them will be found to have malignant ovarian masses approximated 10% of the population (3). It is believed that an ovarian malignancy to be found in female patients of less than 15 years of age is account for only 1% of all population(4).
On other hand, while the discovering of adnexia mass in a preschoolar and adolescent are taking much concerned warranted towards the plan of conservative management because the majority of these adnexia mass are of benign in original (5).
The only important aspect which is needed during the surgical approach is to be aware of its presentation the mode of evaluating and the treatment approach in order to attain a successful conservation of normal ovarian function (6).
Actually the histopathological findings of adnexia masses are categorized into functional cysts, benign neoplasms and malignant neoplasms. It has been observed that preschoolar and adolescent there are at risk of developing functional ovarian cyst which is due to failure of involution of follicles commonly caused by gonadotropin stimulation of the ovary by the immature hypothalamic-pituitary axis (7). The reviews found that ovarian cysts among prepubertal and adolescent undergoing routine ultrasound were only 2 to 5 percents and most of them had size of less than 1cm (8). The findings were insignificant among post pubertal adolescent whereby the reasons of ovarian cyst was due to the failure of ovulation or persistence of ovarian follicles. However, in a review of female under the age of 21 undergoing surgery for an adnexal mass, 57.9% of the cases were diagnosed with an ovarian cyst (9).
The most of adnexia masses presenting with many ways including abdominal pain, distended and palpable abdominal mass, nausea, vomiting, increasing respiratory discomfort and precocious puberty(10). It has been demonstrated that in the adolescent population with ovarian cysts most likely of its presentation has been associated with irregular menstrual cycles but these feature may be differ among prepubertal females and commonly presents as a palpable abdominal mass (11).
On other hand, a torsion or ruptured haemorrhagic ovarian cyst should be suspected among the patients who present with a sudden onset of severe abdominal pain, fever, nausea, and vomiting (12).
There is controversial regarding the prediction of suspicious ovarian malignancy supported by clinical symptoms only, although increased index of suspicion is warranted for patient who present with an asymptomatic palpable abdominal mass or signs and symptoms of precocious puberty (13). However, age of patients at the time of presenting signs and symptoms may also contributing to pose a significant risk factor for malignancy like our patient, she was 5 years old whereby the risk of developed to malignancy found to be 3 fold higher compared to the patients aged 15 to 19 years old (14).
The challenged concern differential diagnosis of adnexia masses among pre scholar and adolescent could be observed from initial aspect during taking a thorough history, physical examination, evaluating of the genitourinary and gastrointestinal tracts when patients present with abdominal pain or a palpable mass (15). Thereafter, it is important to discuss patterns and history of her menstrual cycle, sexual history, and any uses of any types of contraceptive methods to help elucidate the cause of symptoms.
Again the pregnancy test and a complete blood count should be ordered immediately in patients who present with severe abdominal mass pain to identify the presence of pregnancy, Leukocytosis, anaemia, and haemorrhage. The twisted or torsion ovarian cyst should be Ovarian suspected if fever, severe pain, and Leukocytosis are present (16). The intervention of emergencies surgery due to the ruptured ectopic pregnancy, appendicitis and ovarian torsion should be managed appropriately.
Case Presentation
A 4 year-old pre-pubertal girl presented with history of rapidly abdominal distension and fever for one month. She was apparently well until one month prior to admission when she developed abdominal distension. It started gradually and progressively rapidly increasing with time. The distension was associated with reduced appetite, but it was not associated with nausea, vomiting, abdominal pain, diarrhea or constipation. She also reported to have several episodes of difficulty in breathing on lying flat and non-productive cough, but there was no history of chest pain, awareness of heart beat, blurred vision, dizziness and loss of consciousness. She also reported history of fever for one month prior to admission. She experienced low grade fever with no specific periodicity.
It was temporarily relieved by panadol. However, there was no history of night sweats or weight loss reported. She had neither history of headache nor convulsions during the course of illness. There was history of painless swelling of the face and lower limbs, although reported to have been walking properly. The baby was also reported to have decreased urine output, but there was no history of painful micturation. There was neither history of vaginal bleeding nor that of breast discharge but, with time, she noticed non tender breast engorgement.
This was her second admission; the first was at the age of 2 years, due to malaria. She was treated and discharged home in good condition. Pre natal, natal and postnatal history. There were no complications reported throughout her pregnancy, labour and puerperium.
The developmental milestones were attained accordingly. She was sitting without support at 4 months, crawled and said word‘her earliest native language’ at 8 months and walked on support at 12 months. At the time of admission she was able to talk properly, obey commands and socialize with other family members. Her developmental milestones were consistence with a 4 year old child. The baby received all immunization as per Tanzanian Expanded Program of Immunization. She was a third born in a family of four children. Other siblings were doing fine and both parents were living together. Her parents worked as peasants. There was no familial history of childhood cancers, sickle cell or kidney diseases.
General examination she was alert, had generalized mild facial and lower limb non pitting oedema to the level of the knee bilaterally. Otherwise she was not dehydrated, not pale, not jaundiced and Afebrile (Temperature was 36.5°C). Other vital signs: pulse rate of 130 beats per minute, respiratory rate of 36 cycles per minute.
Anthropometric measurements were as follows: weight of 13kg, height of 98 cm and mid-upper arm circumference of 13cm which were normal for age as per WHO standard charts (She was not malnourished). Abdominal examination was tense, shinny, symmetrically grossly distended and it was moving with respiration. The umbilicus was everted, but there were no therapeutic scars or distended veins. There was no tenderness elicited on palpation. It was difficult to examine for the abdominal organs due to gross distension. The entire abdomen was dull on percussion with positive fluid thrill. The bowel sounds were audible and there were no added sounds (Figure 1).
Cardiovascular system examination revealed radial pulse 130 beats per minute, regular, with strong volume and synchronized with other peripheral pulses. The jugular venous pressure was not raised. There was no precodial hyperactivity. The apex beat was palpable at the fifth inter-costal space in the mid clavicular line. The first and second heart sounds were heard with no murmurs. Respiratory system examination she was dyspnoeic on lying flat, but normal on sitting. The trachea was central, and chest was moving symmetrically with respiration. The chest was resonant on percussion with vesicular breath sounds heard on auscultation. There were no added sounds.
Pelvic vaginal examination she was found to have moderately edematous labia majora, which were non tender. The labia minora, hymen and clitoris were normal in appearance. She had neither bleeding nor discharge from the vaginal introitus and the mons pubis looked normal for her age. Examination of the central nervous and musculoskeletal systems was unremarkable. She had working diagnosis of ovarian Tumor with various differential diagnosis includes Extra pulmonary Tuberculous of peritonitis and other intra-abdominal tumors (Kidney, liver, bladder). Investigations findings was hormonal assays: Alpha-fetoprotein(AFP) found to be high range 10.0ng/mL with reference ranges < 8.5ng/Ml, Follicle stimulating hormone(FSH) found to be normal 2.0mIU/L with the reference range 0.0-2.8mIU/L and Human Chorionic Gonadotrophin found to be high 5IU/L with reference range <3.0 IU/L.
Serum biochemistry results Low Total protein 53.5g/dl Reference range 60-80g/dl, Low Albumin27.08g/dl Reference range 36.00-47.00g/dl, normal Direct Bilirubin 0.0µmol/l reference range of 0-5.00µmol/l, low Urea/BUN 2.22mmol reference range 3.00-8.30, level of Alanine AminoTransferase (ALT) 14.86U/L reference range of 0-50.0U/L, normal level of Aspartate AminoTransferase (ASAT) 38.26U/L range reference 0-50.0U/L.
Hematology Results: Normal Haemoglobin 11.6 g/dl reference range of 11.0-13.6g/dl, Packed Cell Volume 41.8% reference range of 36-44%, Red Blood cell count reference range of ,Mean cell volume79.3fl reference range of 76-96fl , low Mean cell haemoglobin 22.0pg reference range of 27-32pg , low Mean cell haemoglobin concentration 27.7% reference range of 30-35% ,White blood cell count 7.54x109 /L reference range of 4.0-11.0x10/L, Differentials: Neutrophils43.2% reference range of 40.0-75.0%, High Lymphocytes 47.8% reference range of 25.0-45%, Monocytes 7.5% reference range of 3.0-10.0%, Eosinophil 0.2% reference range of 0-6.0%, Basophils 0.3% reference range of 0.0-5.0% , Platelets 414x109/L reference range of 150-400x109/l.
Miscellaneous investigations includes the following normal stool analysis, Non reactive MRDT and no parasite seen Blood slide for malaria parasites, HIV Serology Negative, Blood type O, Rhesus positive, Chest X-ray Normal cardiac shadow, clear lung fields bilaterally, no features of metastasis, Abdominal pelvic ultrasound found a solid hyper echoic mass, measuring 8x6cm was seen in the right ovary. The Uterus, liver, kidneys, pancreases and spleen were normal. Massive Intraperitoneal fluid was seen. Conclusion was ovarian tumour suspected of germ cell type.
The child was prepared for cyto reductive surgery after received informed consent her mother other closely family member on behalf. Three units of standby blood transfusion were prepared. Pre operative diagnosis was a Malignant Ovarian tumour and probable Germ cell tumour. She was operated under general anaesthesia with endotracheal intubation. Induction of anaesthesia was done using Suxomethonium and morphine. She was maintained on halothane, morphine, Pancuronium and oxygen. The patient was positioned on supine then aseptically cleaned and draped. Abdomen was opened through right extended low paramedian incision.
Intra operative findings were amassive haemorrhagic ascitic fluid measured 3000mls found collected intraperitoneal cavity that was due to the ruptured left ovarian tumor which was cauliflowers appearance no extensive features of malignancy idenfied. The Right ovary, both oviducts and uterus appeared normal. The omentum, liver, spleen and bowels appeared normal with no signs of metastatic deposits.
Done was a left oophorectomy and suctioning of all ascitic fluid. The removed ovary and ascitic fluid was sent for histocytopathological analysis. The abdomen was closed in layers with vicrly number 1 for rectus sheath and monofilament nylon number 3/0 for skin. Intra-operatively she received prophylactic intravenous metronidazole 250mg and gentamycin 40mg (Figure 2).
Progress post operative, patient was admitted in the intensive care unit 24 hours for close observation and monitoring. She was given intramuscular pethidine 50mg for 6hourly and maintenance by Ringer lactated of 1000ls for 24 hours. On the second day she was transferred to the gynaecological ward and started on oral sips. Post-operative period was uneventful and the child was discharged on the seventh day in good condition.
She was follow up as outpatientat the gynaecological clinic five weeks post discharge and at that time the cyto-histopathology results were out. Ascitic fluid analysis was negative for Acid Fast bacilli staining and no bacterial and tuberculous growth was seen on culture. The final diagnosis after histopathology results was ovarian malignant Endodermal sinus tumour (Yolk sac tumor) with FIGO Stage IC. The patient was thus referred to the medical oncology department for possible adjuvant chemotherapy whereby she was planned for chemotherapy with cisplastin and Etoposide but she was made a two subsequent visits at the oncology clinic were pre-chemotherapy work-up was ordered and planned to start her chemotherapy management on three-weekly basis a cisplastin and Etoposide, but she lost follow up.
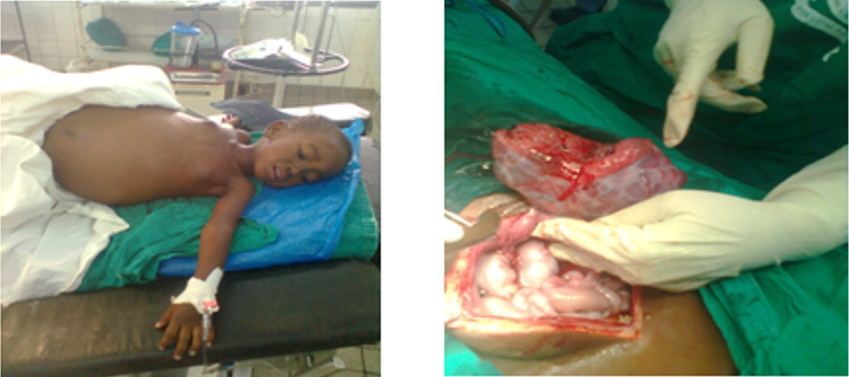
Figure 1: Preparation before operation of the preschooler girl done in the operating theatre.
Figure 2: Intra operative finds that a source of distended abdomen was due the accumulation of cystic fluids mixed with blood from ruptured left ovarian cyst.
Figure 2: Intra operative finds that a source of distended abdomen was due the accumulation of cystic fluids mixed with blood from ruptured left ovarian cyst.
Discussion
Ovarian tumors are the most common gynaecological malignancy occurring in childhood. Germ cell tumors are the leading ovarian tumours in children, unlike in adults where epithelial tumours dominate. Ovarian germ cell neoplasms are derived from primordial germ cells of the ovary and they comprise about a quarter of ovarian neoplasms overall (17).
Endodermal sinus (Yolk sac) tumors accounts for 20% of the malignant ovarian germ cell tumor and thus making it the second after Dysgerminoma. Median age at presentation is 18 to 25 years and they have been rarely described in pre menarchal girls (18). The cause of this adnexia mass is still unknown although risk factors such as exposure to maternal exogenous hormone or X-rays during pregnancy have been suggested (19).
Malignant yolk sac tumors progresses very fast and the median duration of symptoms are usually 2 to 4 weeks. Abdominal distension and pain are the most common presenting features, although in our case pain might have been obscured by prolonged course of paracetamol prescribed for persistent fever (20). Low grade fever in these cases may result from effect of pyrogenic cytokines (IL-1, IL-6, TNF, IFN) produced by the tumor.
Ascites is present in 20% of the cases and it might result from rupture of the tumor during or before surgery as it is occur in our case the cystic ovary had rupture and leakage in the peritoneal leading of massive ascites (21).
Alpha-feto-protein is commonly elevated in yolk sac tumors and thus utilized as a reliable diagnostic, monitoring and prognostic marker. Elevation of Human Chorionic gonadotrophin (HCG) marks the germ cell origin of this tumor, although it is mostly noted in non gynaecological cause of choriocarcinoma (22). Endocrine manifestations such as menstrual irregularities, hirsutism and precocious puberty due to the influence of hormones secreted by the tumor have also been described (23).
The diagnosis and staging of ovarian tumors is normally completed at explorative laparotomy, although histological typing relies on cyto-pathological analysis of the resected tumor and ascitic fluid. Nearly two thirds of the Yolk sac tumors are diagnosed at FIGO stages I or II, and they are usually unilateral due to their rapid progression (24).
Explorative laparotomy was mandatory in this case for both diagnosis and treatment. The optimal cytoreductive or Debulking surgery has to be performed because it has impact on prognosis (25). Unilateral oophorectomy was done in our patient because at such time she was at very young age which she could need preserved of her future fertility and was highly encouraged, especially when there no features of metastasis malignancy (26).
The loss of opportunity to have adjuvant chemotherapy in our case might lead to poor prognosis simply because attempting surgery alone has the inadequate better prognosis. However, it should be remembered that, Yolk sac and other germ cell tumors are highly chemo-sensitive, especially to platinum based combination of Bleomycin, Etoposide, and cisplastin (BEP) whereby an excellent outcomes have been reported (27).
Yolk sac tumours of the ovary are relatively uncommon neoplasms characterized by rapid progression. Majority of cases are diagnosed at 15-25 years of age, but they can also occur in pre-menarchal girls as it occur in our case (28).
Alpha-feto-protein is a reliable diagnostic, monitoring and prognostic marker, although complete diagnosis and staging is achieved at explorative laparotomy. Treatment should be accomplished by adjuvant platinum-based chemotherapy after optimum cyto-reductive surgery (29).
Conclusion
Endodermal sinus (yolk sac) is a very rare tumor typically affects girls and young women. Invasive and non invasive approach will remain to be standard goal of managements. Delayed on approaching the definitive management may encounter the serious complication when it comes up to already in advance stage. The presentation of an adnexal mass in preschoolar girls is a concerning event for the health care givers, patient and the family.
References
- Hee Heo S, Woong Kim J, Soo Shin S, In Jeong S, Soon Lim H, Duk Choi Y, et al. (2014). Review of Ovarian Tumors in Chil- dren and Adolescents: Radiologic- Pathologic Correlation 1. RadioGraphics. 34(5):2039–55.
- Zolton J, Maseelall P. (2013). Evaluation of ovarian cysts in adolescents. Open J Obstet Gynecol. 2013 (September):12–6.
- Eskander RN, Bristow RE. (2012). Adnexal Masses in Pediatric and Adolescent Females: A Review of the Literature. Curr Obstet Gynecol Rep. 1(1): 25–32.
- Rathore R, Sharma S, Arora D, Arora D. (2016). Spectrum of Childhood and Adolescent Ovarian Tumors in India: 25 Years Experience at a Single Institution. Open Access Maced J Med Sci. 4(4): 551–5.
- Timur ES, Incebiyik A, Camuzcuoglu A, Hilali NG, Camuzcuoglu H, Vurat M. (2015). Adnexal mass requiring surgical intervention in adolescent girls. eEuropian Jouranl Gen Medcine. 12(3): 239–43.
- Choudry A, Bangash N, Malik A, Choudry H. (2008). Adolescent ovarian tumors: a clinicopathlogical review of 15 cases. J Ayub Med Coll Abbottabad. 20(4): 18–21.
- Kinson MS, Spiryda LB. (2016). Management of Adnexal Masses in Children and Adolescent Populations: Advocating for Ovarian Conservation. Surg Res - Open J. 2(2):72–6.
- Saber MM, Zeeneldin AA, El Gammal MM, Salem SE, Darweesh AD, Abdelaziz AA, et al. (2014). Treatment outcomes of female germ cell tumors: The Egyptian National Cancer Institute experience. J Egypt Natl Canc Inst. 26(2):103–8.
- Low JJH, Ilancheran A. (2012). Best Practice & Research Clinical Obstetrics and Gynaecology Malignant ovarian germ-cell tumours. Best Pract Res Clin Obstet Gynaecol. 26(3): 347–55.
- Yeap ST, Hsiao CC, Hsieh CS, Yu HR, Chen YC, Chuang JH, et al. (2011). Pediatric malignant ovarian tumors: 15 years of experience at a single institution. Pediatr Neonatol. Elsevier Taiwan LLC; 52(3): 140–4.
- Péroux E, Franchi-Abella S, Sainte-Croix D, Canale S, Gauthier F, Martelli H, et al. (2015). Ovarian tumors in children and adolescents: A series of 41 cases. Diagn Interv Imaging. Elsevier Masson SAS; 96(3): 273–82.
- Riman T, Dickman PW, Nilsson S, Correia N, Nordlinder H, Magnusson CM, et al. (2001). Risk factors for epithelial borderline ovarian tumors: Results of a Swedish case - Control study. Gynecol Oncol. 83(3): 575–85.
- Chaopotong P, Therasakvichya S, Leelapatanadit C, Jaishuen A, Kuljarusnont S. (2015). Ovarian Cancer in Children and Adolescents: Treatment and Reproductive Outcomes. Asian Pac J Cancer Prev. 16(11): 4787–90.
- O’Neill KE, Cooper AR. (2011). The Approach to Ovarian Dermoids in Adolescents and Young Women. J Pediatr Adolesc Gynecol. 24(3): 176–80.
- Chang HC, Bhatt S, Dogra VS. (2008). Pearls and Pitfalls in Diagnosis of Ovarian Torsion. RadioGraphics. ;28(5): 1355–68.
- Paltiel HJ, Phelps A. (2014). US of the Pediatric Female Pelvis. Radiology. 270(3): 644–57.
- Zhao T, Liu Y, Zhang H, Wang X, Jiang H, Lu Y. (2016). Malignant ovarian germ cell tumors in adolescents?: 18 years of experience at a single institution. Int J Clin Exp Med. 9(6): 11934–41.
- Billmire DF, Vinocur C, Rescorla F, Cushing B, London W, Schlatter M, et al. (2004). Outcome and Staging Evaluation in Malignant Germ Cell Tumors of the Ovary in Children and Adolescents: An Intergroup Study. J Pediatr Surg. 39(3): 424–9.
- Doubeni CA, Doubeni ARB MA. (2016). Diagnosis and Management of Ovarian Disorders. Am Fam Physician. 93(11): 937–44.
- Giardino, A.P., Pasquariello, P.S., Rahhal, R.M., Eddine, A.C., & Bishop WP. (2006). A Child with an Abdominal Mass. Hosp Physician. 42(2): 37–42.
- Ashnagar A, Alavi S, Nilipour Y, Azma R, Falahati F. (2013). Massive ascites as the only sign of ovarian juvenile granulosa cell tumor in an adolescent: a case report and a review of the literature. Case Rep Oncol Med. 386725.
- Forstner R, Kinkel K. (2007). Adnexal Masses: Characterization of Benign Ovarian Lesions. MRI CT Female Pelvis. 197–232.
- Rosenfield RL, Ehrmann DA, Littlejohn EE. (2015). Adolescent polycystic ovary syndrome due to functional ovarian hyperandrogenism persists into adulthood. J Clin Endocrinol Metab. 100(4): 1537–43.
- Ki EY, Byun SW, Choi YJ, Lee KH, Park JS, Lee SJ, et al. (2013). Clinicopathologic review of ovarian masses in korean premenarchal girls. Int J Med Sci. 10(8): 1061–7.
- Schorge JO, Clark RM, Lee SI, Penson RT. (2014). Primary debulking surgery for advanced ovarian cancer: Are you a believer or a dissenter? Gynecol Oncol. 135(3): 595–605.
- Pérez-López FR, Ceausu I, Depypere H, Kehoe S, Lambrinoudaki I, Mueck A, et al. (2017). Interventions to reduce the risk of ovarian and fallopian tube cancer: A European Menopause and Andropause Society Postition Statement. Maturitas. 100: 86–91.
- Towne BH, Hossein Mahour G, Woolley MM, Isaacs H. (1975). Ovarian cysts and tumors in infancy and childhood. J Pediatr Surg. 10(3): 308–9.
- Surgery P, Hospital BYLN, Central M. (2017). OVARIAN YOLK SAC TUMOUR IN A GIRL ? CASE REPORT. Develepmental Period Med. 101–3.
- Chang YW, Chao KC, Sung PL, Li WH, Wang PH. (2013). Treatment of ovarian endodermal sinus tumor to preserve fertility. J Chinese Med Assoc. 76(2): 112–4.
Citation: Athanase Lilungulu., et al. (2020). A Case of Preschoolar Girl Presented with Progressive Huge Distended Abdominal Diagnosed to be Huge Ovarian Cyst Managed by Explorative Laparotomy: Histopathology Revealed Endodermal Sinus (yolk sac) Ovarian Tumour. Journal of Gynaecology and Paediatric Care 2(2).
Copyright: © 2020 Athanase Lilungulu. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
