Research Article
Volume 5 Issue 3 - 2023
Assessment of Heavy metals in Shrimps; Penaeus notialis, Penaeus Monodon, and Prawn; Macrobrachium Macrobrachion Sampled from three [3] water Bodies in Lagos State, Nigeria: An Inimical to Human Health Safety Issues
Department of Fisheries, Faculty of Science, Lagos State University, Ojo, Lagos, Nigeria
*Corresponding Author: Adeboyejo O. Akintade, Department of Fisheries, Faculty of Science, Lagos State University, Ojo, Lagos, Nigeria.
Received: September 01, 2023; Published: September 16, 2023
Abstract
To generate baseline data on heavy metals in shrimps landed in Local fishing communities and provide detailed reports on their inimical human health issues. Seven (7) metals were studied between January and July 2021 to assess their concentrations (Arsenic, Cobalt, Chromium, Cadmium, Lead, Magnesium, and Nickel) in Shrimps, Penaeus notialis, Penaeus monodon, and prawn, Macrobrachium macrobrachion sampled from three [3] water bodies in Lagos state, Nigeria (including Ologe lagoon (SS1), Badagry creek (SS2), and Lagos lagoon (SS3). A total of 162 samples were selected and the concentration of heavy metals was determined by Atomic Absorption Spectrometry (AAS) method, following electrothermal heater digestion. Na (335.8±48.9), PO3 (111.4±31.7), and As. (0.44±0.25) in M. macrobrachion were higher in Ologe lagoon than in other locations. In P. monodon, Ni (0.04±0.07), Zn (1.78±2.8), SO4 (55.05±9.2), cobalt (0.08±0.07), iron (4.39±2.39) and Cd (0.1±0.13) were higher in Lagos lagoon. While P. notialis alsohad higher values in Mn (1.66±1.2), Ni (0.045±0.09), Zn (1.33±1.36), SO4 (55.07±11.9), Cd (0.05±.0.1) and Fe (4.17±2.13) in Lagos lagoon than other sample stations. The study revealed significant differences at P<0.05 in mean concentrations of heavy metals across all sample stations which are found below the permissible level recommended by WHO for human consumption. Nevertheless, there were probabilities of the human health risk posed by levels found, due to biomagnification along the food chain.
Keywords: Human health; Water quality; Heavy Metals; Shrimps & Prawns
Introduction
Globally, freshwater decapods have been one of the major food delicacies because of their rich deposits of minerals, metals, nutrients, protein, fibers, and cellulose. High levels of mineral contents like metals are usually accumulated in the body tissues of these organisms because of their lifestyle. This has necessitated the increased rate of human consumption in recent times (Anani and Olomukoro, 2018). The bioaccumulation/biomagnification of heavy metals in living organisms describes the processes and pathways of pollutants from one trophic level to another (Akan et al., 2012). The prevailing conditions of the aquatic environment could cause free divalent ions of some heavy metals to be absorbed by the gills and skin of fish (Part et al., 1985). Heavy metals constitute one of the most dangerous groups of water pollutants because of their persistent nature, toxicity, and tendency to accumulate in organisms and become transferred from one trophic level to another in the food chain (Alinnor et al., 2016).
The concentration of heavy metals in fish organs indicates that the aquatic ecosystem is polluted and a measure of food safety (Farkas et al., 2000). Fish are the most important organisms in the aquatic food chain and are sensitive to heavy metal contamination which has lethal and chronic effects on them (Akan et al., 2012). Amongst the different organs, the liver accumulates higher concentrations of metals and has been used widely to investigate the process of bioaccumulation while the kidneys play a vital role in the excretion of heavy metal ions (Wepener et al., 2001). Heavy metals can be classified as potentially toxic; (such as arsenic, cadmium, lead, and mercury), probably essential; (such as nickel, vanadium, cobalt) and essential; (such as copper, zinc, iron, and manganese) according to Kheradmand, et al. (2006).
Heavy metals cause neurological disorders, hormone imbalances, cardiovascular failure, kidney ailments, infertility, hair loss, endocrine disorders, respiratory and digestive issues, and cancer when they accumulate in the body even at trace levels. (Islam, et al, 2017). A variety of industrial activities discharge their wastewater into the rivers, where it settles and enriches river sediments. Heavy metal pollution is normally assessed using river sediments and a set of specified pollution indices (Ganugapenta et al., 2018). Metal accumulation in freshwater decapods has been acclaimed and perceived to cause serious health concerns when transferred to humans along the food chain. Environmental valuation of the noxiousness of metals in the freshwater Sudanonautes africanus and Macrobrachium rosenbergii had been shown to have probable human health hazard effect concomitant by way of ingestion (Anani and Olomukoro, 2018). Health risks associated with heavy metals such as renal failure, skeletal deformation, and hepatic failure have been linked to their non-decomposable and persistent nature in the visceral of humans (Duruibe et al., 2007). This can lead to severe maladies like dysentery, stomach aches, head-tremor, anaemia, paralysis, nausea, paroxysm, melancholy, and even respiratory disorders which can be either acute or chronic forms; neuron toxicity, oncogenic, genetic alteration, or teratogenicity (European Union, 2002).
This study aims to determine the level of heavy metals bioaccumulation in Shrimp and prawns from three fishing communities in Lagos state, Nigeria; with a view to assessing the health risk/safety of these Shellfishes for human consumption. To ensure that this information is important for decision-making on sustainable development within the Lagos water complex. To ensure that information gathered will contribute to literature for other scholars with interests in research on heavy metal pollution.
Materials and Methods
Study Area and Geographic Settings
Three sampling stations were selected for the study: Ologe lagoon [SS1], Badagry creek [SS2], and Lagos lagoon [SS3]. The choice of the three sampling stations was based on their accessibility to aquatic ecosystems which has biomonitoring organisms (Macrobrachium macrobrachion, Penaeus notialis, andP. monodon), the proximity to an industrial establishment, human activities, and suitability for a future survey. As shown in figure 1; Lagos State is situated in the southwestern part of Nigeria on the narrow coastal floodplain of the Bight of Benin. It lies approximately between latitude 6°22’N and 6°42’N and 3°22’E and between longitude 2°42’E. It is bounded in the North and East by the Ogun State of Nigeria, West by the Republic of Benin, and South by the Atlantic Ocean. Lagos stretches for 180 km along the coast of the Atlantic Ocean. The dominant vegetation of the State is the tropical swamp forest consisting of marine, brackish and freshwater ecological zones with varying species that provide productive fishing opportunities.
Three sampling stations were selected for the study: Ologe lagoon [SS1], Badagry creek [SS2], and Lagos lagoon [SS3]. The choice of the three sampling stations was based on their accessibility to aquatic ecosystems which has biomonitoring organisms (Macrobrachium macrobrachion, Penaeus notialis, andP. monodon), the proximity to an industrial establishment, human activities, and suitability for a future survey. As shown in figure 1; Lagos State is situated in the southwestern part of Nigeria on the narrow coastal floodplain of the Bight of Benin. It lies approximately between latitude 6°22’N and 6°42’N and 3°22’E and between longitude 2°42’E. It is bounded in the North and East by the Ogun State of Nigeria, West by the Republic of Benin, and South by the Atlantic Ocean. Lagos stretches for 180 km along the coast of the Atlantic Ocean. The dominant vegetation of the State is the tropical swamp forest consisting of marine, brackish and freshwater ecological zones with varying species that provide productive fishing opportunities.
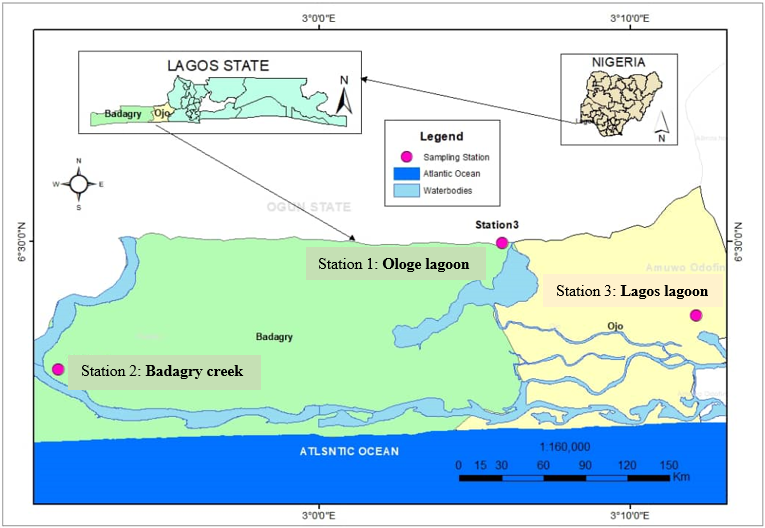
Figure 1: Geographical map of the Lagos state lagoons and creeks, inset the sampling areas: Ologe lagoon (SS1), Badagry creek (SS2) and Lagos lagoon (SS3).
Sample collection and preparation
The sampling duration was from November 2021 to May 2022. The sampling stations were visited monthly throughout the study period. Prawn and shrimp samples were collected in the early hours of the morning between 8am and 10am, after the arrival of fishermen at the respective locations. Prawn and shrimp samples were purchased at random across the various areas and preserved in ice and taken to the laboratory for identification and analysis. They were kept frozen in the refrigerator pending heavy metals analysis in the laboratory.
The sampling duration was from November 2021 to May 2022. The sampling stations were visited monthly throughout the study period. Prawn and shrimp samples were collected in the early hours of the morning between 8am and 10am, after the arrival of fishermen at the respective locations. Prawn and shrimp samples were purchased at random across the various areas and preserved in ice and taken to the laboratory for identification and analysis. They were kept frozen in the refrigerator pending heavy metals analysis in the laboratory.
Sample digestion
Dry samples of gill, muscle, and the liver (0.1g) were ground to powder using mortar. The samples were then transferred into a digestion flask containing a mixture of concentrated sulphuric acid (H2SO4) and concentrated nitric acid prepared in a 3:1 (v/v) ratio and heated in a water bath. The heating was followed by the repeated addition of ¾ drop of hydrogen peroxide (H2O2) until the solution became clear. Hydrogen peroxide was added to reduce nitrous vapour and accelerate digestion by raising the temperature (Saha et al., 2016). After 20 minutes of additional heating at 150°C, the samples were allowed to cool to room temperature. The gills and muscle samples were diluted with deionized water up to 50ml and 25ml for liver samples. The diluted samples were filtered through No.1 Whatman filter paper, and the filtrate is made up to 25ml with distilled water (Thomas and Mohaideen, 2015).
Dry samples of gill, muscle, and the liver (0.1g) were ground to powder using mortar. The samples were then transferred into a digestion flask containing a mixture of concentrated sulphuric acid (H2SO4) and concentrated nitric acid prepared in a 3:1 (v/v) ratio and heated in a water bath. The heating was followed by the repeated addition of ¾ drop of hydrogen peroxide (H2O2) until the solution became clear. Hydrogen peroxide was added to reduce nitrous vapour and accelerate digestion by raising the temperature (Saha et al., 2016). After 20 minutes of additional heating at 150°C, the samples were allowed to cool to room temperature. The gills and muscle samples were diluted with deionized water up to 50ml and 25ml for liver samples. The diluted samples were filtered through No.1 Whatman filter paper, and the filtrate is made up to 25ml with distilled water (Thomas and Mohaideen, 2015).
Determination of Heavy Metals
To determine the metals in the tissues of the samples, an atomic absorption spectrometer (AAS) was used. All chemical reagents were graded (Merck). The glassware and plastic containers were acid-washed with 10% nitric acid and rinsed twice with distilled water before use and the results were expressed in mg/kg dry weight of the samples. The resultant filtrates obtained from the digestion process were subject to the Atomic Absorption Spectroscopic Analysis (AAS) of heavy metals. The concentration of Arsenic, Cobalt, Chromium, Cadmium, Lead, Manganese, and Nickel were measured in all the samples on the PG instrument (AA990) AAS that uses air-acetylene flame with UV-detector and automatic zero to compensate the blank. A hydride generator was used along with the flame AAS for Arsenic detection. The calibration curves for each standard were prepared individually by applying linear correlation using the least square method.
To determine the metals in the tissues of the samples, an atomic absorption spectrometer (AAS) was used. All chemical reagents were graded (Merck). The glassware and plastic containers were acid-washed with 10% nitric acid and rinsed twice with distilled water before use and the results were expressed in mg/kg dry weight of the samples. The resultant filtrates obtained from the digestion process were subject to the Atomic Absorption Spectroscopic Analysis (AAS) of heavy metals. The concentration of Arsenic, Cobalt, Chromium, Cadmium, Lead, Manganese, and Nickel were measured in all the samples on the PG instrument (AA990) AAS that uses air-acetylene flame with UV-detector and automatic zero to compensate the blank. A hydride generator was used along with the flame AAS for Arsenic detection. The calibration curves for each standard were prepared individually by applying linear correlation using the least square method.
Statistical data for analysis
Data were analysed and presented using Microsoft Excel 2010 and IBM SPSS Statistics software programmes to calculate mean ± standard deviation. All the results expressed as the mean of each sample measured. To evaluate the statistical differences between samples, one way ANOVA was adopted. Significance was established at P>0.05. A further study to determine the species showing significant disparities was done using a Duncan Multiple Post Hoc Test.
Data were analysed and presented using Microsoft Excel 2010 and IBM SPSS Statistics software programmes to calculate mean ± standard deviation. All the results expressed as the mean of each sample measured. To evaluate the statistical differences between samples, one way ANOVA was adopted. Significance was established at P>0.05. A further study to determine the species showing significant disparities was done using a Duncan Multiple Post Hoc Test.
Results
This study evaluates the heavy metals concentration of Macrobrachium macrobrachion, Penaeus monodon and P. notialis from three fishing communities: Ologe lagoon, Badagry creek and Lagos lagoon. The results of the heavy metals analyses (such as Magnesium, Sodium, Lead, Cadmium, Chromium, Zinc and Copper) of M. macrobrachion samples collected from these markets were in table 1, the latter part of this chapter includes the result on the investigation of heavy metal bioaccumulation in P. monodon and P. notialis. Table 2 depicts the mean values of M. macrobrachion from Agbara, Badagry and Lagos market. All the analysed heavy metals parameters evaluated were detected.
Variation of heavy metals (Lead and Nickel) was observed. The lead content of 0.21±0.19mg/kg was obtained from Agbara shrimp samples, the value of 0.83±0.78 mg/kg and 0.39±0.36 mg/kg were obtained in shrimp samples from Badagry and Lagos market respectively. M. macrobrachion samples from Agbara market had the highest concentration of calcium (216.25±31.02) mg/kg while the lowest (205.8±37.30) mg/kg was detected in samples from Badagry market. A significant difference (P < 0.05) exists for calcium contents across all sampled stations. The highest concentration of Arsenic was found in shrimp samples from Agbara market (0.44 mg/kg) while the lowest at Lagos market (0.03 mg/kg). The Cadmium values ranged from 0.05±0.04 mg/kg to 0.24 ± 0.23 mg/kg as being depicted below. Sample from Badagry market had the highest concentration of Chromium while the lowest was obtained from shrimp samples from Lagos market. Highest copper and Iron contents were recorded for shrimp sample from Lagos market. It can be seen that cobalt was detected in varying concentrations across the sampling areas. Significant difference (P < 0.05) was established for all the heavy metals evaluated in the study areas.
| Heavy Metals | W.H.O guideline limit |
| Manganese (Mn) | 2.50mg/kg |
| Lead (Pb) | 1ppm |
| Nickel (Ni) | 0.07ppm |
| Zinc (Zn) | 40mg/kg |
| Cadmium (Cd) | 1.0mg/100g |
| Calcium (Ca) | 217mg/100g |
| Chromium (Cr) | 0.05mg/kg |
| Copper (Cu) | 1.0–3.0 mg/100g |
Table 1: World Health Organization guidelines for heavy metals.
| Parameters | Ologe Lagoon | Badagry Creek | Lagos Lagoon |
| Sodium (mg/kg) | 335.79 ± 48.97a | 175.83 ± 42.48b | 217.66 ± 33.87c |
| Potassium (mg/kg) | 111.40 ± 31.70a | 97.04 ± 39.85b | 89.05 ± 25.69c |
| Manganese (mg/kg) | 2.92 ± 1.56a | 3.14 ± 1.95a | 2.32 ± 1.12b |
| Lead (ppm) | 0.21 ± 0.19a | 0.83 ± 0.78b | 0.39 ± 0.36c |
| Nickel (ppm) | 0.03 ± 0.03a | 0.02 ± 0.02a | 0.12 ± 0.19b |
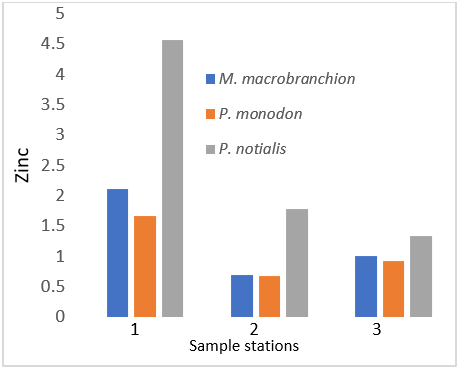
| Zinc (ppm) | 2.11 ± 1.51a | 1.66 ± 0.58b | 4.57 ± 4.32c |
| Sulphate (mg/kg, SO42-) | 55.82 ± 13.57a | 65.79 ± 14.43b | 81.95 ± 13.60c |
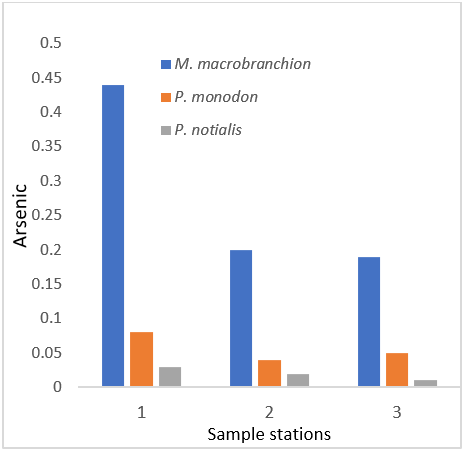
| Arsenic (ppm) | 0.44 ± 0.25a | 0.08 ± 0.11b | 0.03 ± 0.03c |
| Cadmium (ppm) | 0.05 ± 0.04a | 0.05 ± 0.04a | 0.24 ± 0.23b |
| Calcium (ppm) | 216.25 ± 31.02a | 205.8 ± 37.30b | 237.01 ± 24.38c |
| Chromium (ppm) | 0.10 ± 0.16a | 0.11 ± 0.20a | 0.09 ± 0.14a |
| Copper (ppm) | 1.14 ± 0.65a | 0.56 ± 0.59b | 1.73 ± 2.26c |
| Cobalt (ppm) | 0.06 ± 0.05a | 0.027 ± 0.025b | 0.14 ± 0.14c |
| Iron (ppm) | 5.29 ± 2.44a | 5.62 ± 3.54a | 10.16 ± 3.27b |
Note: Mean± SD values are of duplicate determinations. Values in the same row followed by the same letter are not significantly different at P<0.05
Table 2: Heavy metals bioaccumulation in M. macrobrachium from three commercial markets in Lagos state.
Table 2: Heavy metals bioaccumulation in M. macrobrachium from three commercial markets in Lagos state.
Table 3 depicts the mean values of P. monodon from Ologe lagoon, Badagry creek and Lagos lagoon. The lead content of 0.21±0.19mg/kg was obtained from Ologe shrimp samples, the value of 0.83±0.78 mg/kg and 0.39±0.36 mg/kg were obtained in shrimp samples from Badagry and Lagos respectively. P. monodon samples from Ologe had the highest concentration of calcium (216.25±31.02) mg/kg while the lowest (205.8±37.30) mg/kg was detected in samples from Badagry. A significant difference (P < 0.05) exists for calcium contents across all sampled stations. The highest concentration of Arsenic was found in shrimp samples from Ologe lagoon (0.44 mg/kg) while the lowest at Lagos lagoon (0.03 mg/kg). The Cadmium values ranged from 0.05±0.04 mg/kg to 0.24±0.23 mg/kg as being depicted below. Sample from Badagry creek had the highest concentration of Chromium while the lowest was obtained from shrimp samples from Lagos lagoon. Highest copper and Iron contents were recorded for shrimp sample from Lagos lagoon. It can be seen that cobalt was detected in varying concentrations across the sampling areas. Significant difference (P < 0.05) was established for all the heavy metals evaluated in the study areas.
| Parameters | Ologe Lagoon | Badagry Creek | Lagos Lagoon |
| Sodium (mg/kg) | 246.84 ± 83.74a | 137.38 ± 55.54b | 141.10 ± 25.4c |
| Potassium (mg/kg) | 73.44 ± 28.65a | 88.61 ± 32.5b | 55.37 ± 27.71c |
| Manganese (mg/kg) | 1.80 ± 0.91a | 1.04 ± 0.91b | 1.68 ± 0.8a |
| Lead (ppm) | 0.03 ± 0.03a | 0.22 ± 0.32b | 0.23 ± 0.32b |
| Nickel (ppm) | 0.03 ± 0.03a | 0.02 ± 0.02a | 0.04 ± 0.07b |
| Zinc (ppm) | 0.69 ± 0.47a | 0.68 ± 0.19b | 1.78 ± 2.82b |
| Sulphate (mg/kg, SO42-) | 40.56 ± 22.41a | 34.07 ± 17.6b | 55.05 ± 9.27c |
| Arsenic (ppm) | 0.20 ± 0.21a | 0.04 ± 0.04a | 0.02 ± 0.02a |
| Cadmium (ppm) | 0.02 ± 0.02a | 0.02 ± 0.02a | 0.10 ± 0.13b |
| Calcium (ppm) | 188.19 ± 33.87a | 131.66 ± 39.7b | 142.27 ± 49.83c |
| Chromium (ppm) | 0.03 ± 0.03a | 0.14 ± 0.15a | 0.02 ± 0.02a |
| Copper (ppm) | 0.58 ± 0.45a | 0.32 ± 0.6b | 0.57 ± 1.23a |
| Cobalt (ppm) | 0.02 ± 0.02a | 0.02 ± 0.02a | 0.08 ± 0.07b |
| Iron (ppm) | 2.26 ± 1.53a | 2.94 ± 2.94b | 4.39 ± 2.39c |
Note: Mean ± SD values are of duplicate determinations. Values in the same row followed by the same letter are not significantly different at P<0.05
Table 3: Heavy metals bioaccumulation in P. monodon from three commercial markets in Lagos state.
Table 3: Heavy metals bioaccumulation in P. monodon from three commercial markets in Lagos state.
| Parameters | Ologe Lagoon | Badagry Creek | Lagos Lagoon |
| Sodium (mg/kg) | 234.31 ± 71.8a | 132.11 ± 51.1b | 164.39 ± 36.7c |
| Potassium (mg/kg) | 78.26 ± 27.9a | 70.97 ± 25.8b | 52.87 ± 21.5c |
| Manganese (mg/kg) | 1.45 ± 0.5a | 1.03 ± 0.8b | 1.66 ± 1.2c |
| Lead (ppm) | 0.05 ± 0.06a | 0.27 ± 0.3b | 0.20 ± 0.2c |
| Nickel (ppm) | 0.007 ± 0.01a | 0.02 ± 0.02b | 0.045 ± 0.1c |
| Zinc (ppm) | 1.00 ± 0.5a | 0.92 ± 0.2a | 1.33 ± 1.4b |
| Sulphate (mg/kg, SO42-) | 37.91 ± 18.6a | 32.83 ± 16.4b | 55.07 ± 11.9c |
| Arsenic (ppm) | 0.19 ± 0.5a | 0.05 ± 0.04b | 0.01 ± 0.01c |
| Cadmium (ppm) | 0.01 ± 0.01a | 0.03 ± 0.03a | 0.05 ± 0.01b |
| Calcium (ppm) | 175.68 ± 47.0a | 143.7 ± 53.6b | 156.59 ± 33.01c |
| Chromium (ppm) | 0.02 ± 0.01a | 0.04 ± 0.02a | 0.07 ± 0.05b |
| Copper (ppm) | 0.56 ± 0.45a | 0.39 ± 0.4b | 0.31 ± 0.6c |
| Cobalt (ppm) | 0.02 ± 0.02a | 0.03 ± 0.03b | 0.07 ± 0.02c |
| Iron (ppm) | 2.665 ± 1.8a | 2.51 ± 1.0a | 4.17 ± 2.1b |
Note: Mean± SD values are of duplicate determinations, Values in the same row followed by the same letter are not significantly different at P<0.05
Table 4: Heavy metals bioaccumulation in P. notialis from three commercial markets in Lagos state.
Table 4: Heavy metals bioaccumulation in P. notialis from three commercial markets in Lagos state.
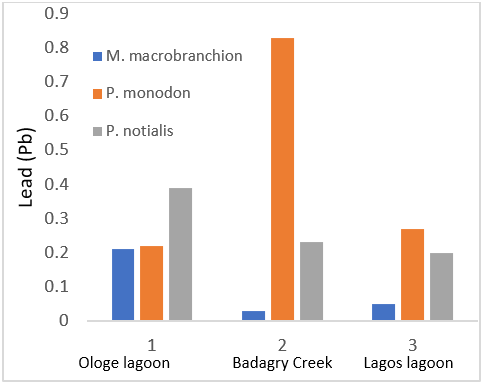
Figure 2: Showing lead (Pb) Concentration in shrimps and prawns from 3 samples stations in Lagos state.
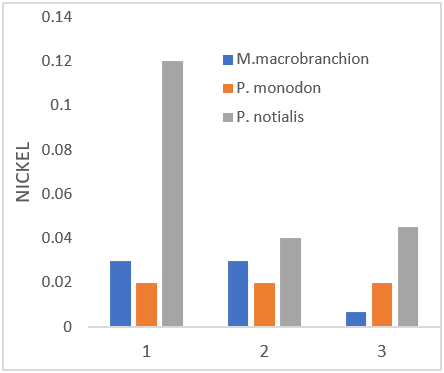
Figure 3: Showing Nickel Concentration in shrimps and prawns from 3 samples stations in Lagos state.
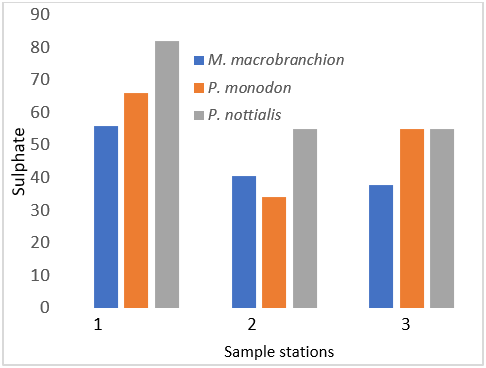
Figure 5: Showing Sulphate Concentration in shrimps and prawns from 3 samples stations in Lagos state.
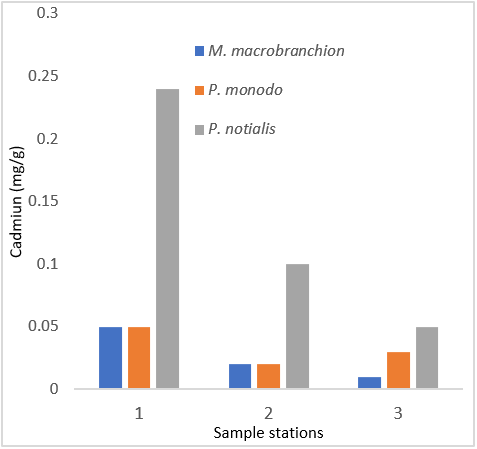
Figure 7: Showing Cadmium Concentration in shrimps and prawns from 3 samples stations in Lagos state.
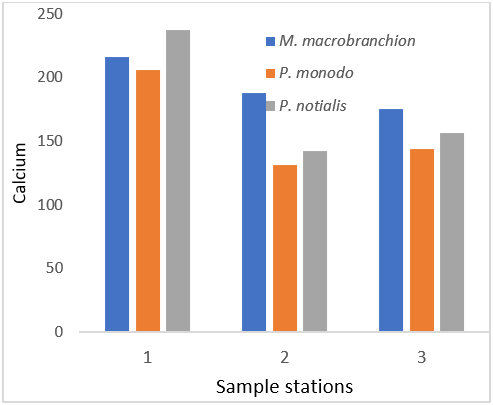
Figure 8: Showing Calcium Concentration in shrimps and prawns from 3 samples stations in Lagos state.
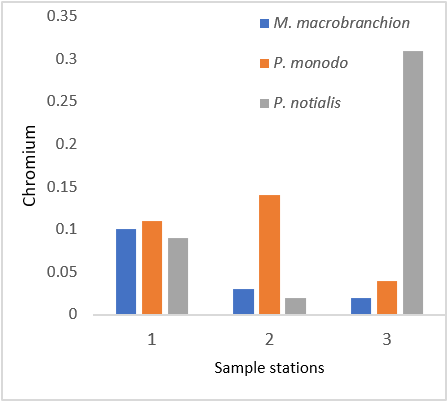
Figure 9: Showing Chromium Concentration in shrimps and prawns from 3 samples stations in Lagos state.
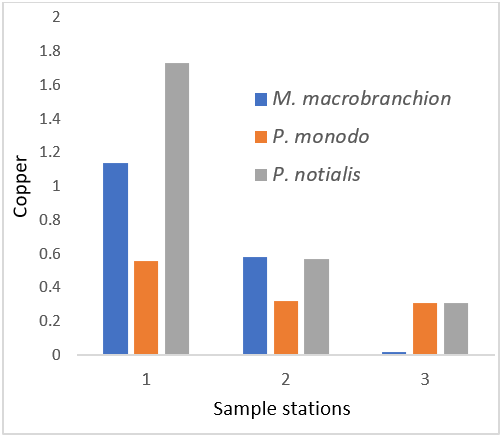
Figure 10: Showing Copper Concentration in shrimps and prawns from 3 samples stations in Lagos state.
Discussion and Conclusion
Heavy metals have been known to accumulate along the trophic levels in freshwater ecosystems. Non-essential metals are not known to play any metabolic function although, as a consequence to their bioaccumulation in fish, these metals can be toxic for humans, even at very low concentrations. The heavy metals concentration in aquatic ecosystem and its invertebrates is important both with respect to nature management and human consumption. Lead (Pb) is a non-essential element for living organism and also possess various adverse effects such as neuro and nephron toxicity, rapid behavioural malfunction, and decreases in growth, metabolism, and survival rate, alteration of social behaviour in some mammals. Lead is known to be toxic and has no known function in biochemical process (Mishra et al., 2010). In the present study, the values of lead are 0.21±0.19mg/100 and 0.83±0.04mg/100 and 0.39±0.36mg/kg in M. macrobrachion from Ologe lagoon, Badagry creek and Lagos lagoon respectively. All the mean Pb concentrations in the fish samples were below the WHO guideline limit of 1 ppm (WHO, 2004). Similar findings have been reported by Olatunji and Osibanjo (2012). The Pb levels in the organs of any aquatic organism could be due to the bioavailability of lead in the aquatic ecosystem from leaded gasoline, industrial effluents and geogenic sources (Akaahan et al., 2010; Akan et al., 2012). From table 1, it can be observed that there is significant difference (P < 0.05) in the concentrations of Lead in the fish samples from different samples stations used in this study.
Copper (Cu) is recognized as essential elements, required by a wide variety of enzymes and other cell components having vital functions in all living things. It play an essential role in haemoglobin biosynthesis, but also causes adverse effects in liver and kidney damage, nausea, acute stomach pains, diarrhoea and fever, etc; if its excessively been ingested. The World Health Organization has listed the estimated safe and adequate daily intake of Cu as 1.0 - 3.0 mg/100 (WHO 2003). Copper accumulation in fish depends upon concentration of the external medium exposure period and the tissue in concern (Kayakar et al., 2010). However, the study revealed that the mean concentration of Cu in samples (M. macrobrachion, P. notialis and P. monodon) which is still below or not exceeding the permissible level recommended by WHO (2003) for human consumption. There was significant difference (P < 0.05) in these samples.
In human nutrition, zinc is an important trace element and fulfils many biochemical functions in human metabolism. A zinc deficiency in human organism leads to several disorders, but an excessive Zn intake can cause acute adverse effects. Zinc (Zn) is critical for aquatic ecosystem and the organisms, including fishes; however, Zn becomes poisonous when it exceeds its maximum value. Zn is a necessary element for embryonic development and important to reproductive organs. The mean concentrations of Zn detected in fresh in samples (M. macrobrachion, P. notialis and P. monodon) were above the permissible of (WHO, 2006). Industrial wastewaters containing zinc stem from galvanic industries, battery production and others might lead to relatively higher amount of zinc in fish. Cadmium is also a toxic element that could be present in aquatic organism especially in fish at high concentrations. An important distribution route is the circulatory system whereas blood vessels are considered to be main stream organs of cadmium toxicity. Chronic exposure to cadmium particulates is generally associated with changes in pulmonary function and chest radiographs that consistent with emphysema. The Cadmium permissible level established by WHO (2003) for fish is 1.0 mg/100g. Comparison with WHO standard also showed that the level of Cd in this study is generally low. Interestingly, elevated concentrations of Cd in aquatic organisms may derive from many anthropogenic sources such as mining, ore treatment, and fertilization.
However, the presence of low Cd level in this location is primarily due to the non- usage of fertilizers for agricultural purposes. Ngo et al. (2005) suggested that commercial phosphate fertilizers which contain small amount of heavy metal contaminations are potential sources of Cd which are delivered to the rivers from upstream areas by runoff during heavy rains and flooding. Cd is a non-essential and highly toxic metal and can induce adverse effects on eel physiology (Lionetto et al., 1998, 2000), especially on reproduction (Pierron et al., 2008). Moreover, the concentrations of Zn in fish may be related to the high concentrations of Cd. Interestingly, positive relationships were observed between these metals in European eel fish samples. Indeed, in animals, Zn protects against Cd injury to animals by competition at the uptake sites and induction of metallothionein synthesis (Hamilton and Mehrle 1986). The weak tolerance of Cd in animals has been found in deficient Zn nutrition (Merian 1991). In some studies, the accumulation of Cd in aquatic organisms has been observed to decrease in the presence of Zn (Elliot et al., 1986). However, exposure to high concentrations of Cd in toxicity experiments has been observed to result in increased accumulations of Zn in tissue of the freshwater fish.
The results of the present study showed that the Cr level was high in all samples except P. notialis from the three sampling stations. These results are not in the same range when compared to WHO permissible limits of 0.05mg/kg. Natural occurrence is in ore, but chromium arises in surface waters from discharges from electroplating, tanning, textile, paint and dyeing plants. Chromium is toxic, to a degree which varies with the form in which it occurs, whether as the trivalent CrIII or the hexavalent CrVI form. The element is an essential dietary requirement in limited amounts and a deficiency can led to disruption of glucose metabolism. Indeed, it has been reported that chromium deficiency is of greater nutritional concern than over-exposure. However, it is considered that the element is carcinogenic (at high concentrations), though much more evidence of this is needed, and it can act as a skin irritant. Nickel is another metallic element which is of moderate concern because of possible carcinogenicity as far as humans are concerned; it also has variable harmful effects on aquatic life. With the presence of Ni in both samples, it should be noted that Ni contamination is a real threat to the aquatic organisms due to their persistence nature and ability to bio-magnify in the aquatic food chain (Ubaid-ullah et al., 2004). The Ni concentration observed in this study were above the permissible limit of WHO guideline limit of 0.07ppm. Ni is however an essential micronutrient in the body of animals (Akan et al., 2012). There was significant difference (P < 0.05) between the Nickel values of all samples. Manganese (Mn) is an essential micro nutrient and functions as a co-factor for many enzyme activities (Suresh et al., 1999). However, high Mn concentration interferes with central nervous system of invertebrates and hence a matter of concern as the consumption of Mn contaminated fish could result to Mn-related disorders in the consumers (Krishna et al., 2014). The Mn concentration levels in fresh samples during the study did not exceed the WHO standard limit of 2.50 mg/kg for fish and fish products (FAO, 2003). There was significant difference (p > 0.05) of manganese concentration levels in the two populations. Iron (Fe) is essential for metabolic reactions and the regulation of cell growth and differentiation; it is also an important constituent of haemoglobin (Oyeleye, 2003). Fe mean levels in all samples were high. The presence of Iron in the fishes could mean that the fishes can play valuable roles in the management of diabetes, which result from insulin malfunction (Wim et al., 2007).
Arsenic recorded low values in all samples which were within the WHO standard. However, it is introduced into water through the dissolution of minerals and ores, from industrial effluents, and from atmospheric deposition: concentrations in ground water in some areas are sometimes elevated as a result of erosion from natural sources. The average daily intake of inorganic arsenic in water is estimated to be similar to that from food; intake from air is negligible." Arsenic is used in the glass and semi-conductor industries and as a fungicide in timber processing. It is very toxic to humans, some arsenical compounds are carcinogens, hence much of the concern regarding them, but there are a variety of other effects on health. The WHO states that inorganic arsenic is a documented human carcinogen, and that a relatively high incidence of skin and possibly other cancers that increase with dose and age has been observed in populations ingesting water containing high concentrations of arsenic.
Furthermore, Calcium in junction with phosphorus, magnesium, vitamin A, C and D, chlorine and protein are all involved in bone formation (Fleck, 1976). Calcium is also important in blood clotting, muscles contraction and in certain enzymes in metabolic processes (Shills, 1973). Magnesium is an important activator of many enzymes system and maintains the electrical potential in nerves. Phosphorus assists calcium in many body reactions, although it has independent functions. Modern diets that are rich in animal proteins and phosphorus may promote the loss of calcium in the urine (Shills and young 1992). This has led to the concept of the calcium to phosphorus ratio. If the calcium to phosphorus ratio is low (low calcium, high phosphorus intake) more than the normal amount of calcium may be lost in the urine thereby decreasing the calcium level in bones. Food is considered “good” if the Ca/P ratio is above 1 and “poor” if the ratio is less than 0.5, while the Ca/P ratio above 2 helps to increase the absorption of cadmium in the small intestine. The result of Ca/P ratio in the fresh and smoked fish samples of present study was greater than 0.5 in favour of phosphorus. This means that both fish samples are rich in highly richly in phosphorus content and are good for consumption since it is within the WHO permissible range of 217mg/100g. Although, high phosphorus content in human body can increase the risk of heart attack, stroke or even death over time. The sodium to potassium ratio of less than 1 is recommended for the prevention of high blood pressure (Nieman et al., 1992). The present study showed that Na/K ratio is 1 to 1.91, 1:1.27 and 1:35 in samples from Ologe lagoon, Badagry creek and Lagos lagoon respectively.
Pattern of heavy metals accumulation from this study are in this order: Zinc >Copper >Manganese >Lead >Chromium>Cobalt >Arsenic >Nickel>Cadmium in fresh samples. The affinity of various metals for fish tissues may differ and be organ-specific, particularly accumulation of essential or bioactive metals such as iron, zinc, copper, or manganese which play important roles in metabolism, physiology and pathology of fish. Heavy metals like zinc, copper and manganese function as a cofactor in several enzyme systems while iron is involved with haemoglobin formation in fish blood (Bury et al., 2003). Accumulation of bioactive metals are actively controlled by the fish through different metabolic processes and the level of accumulation usually independent of ambient concentrations for example, even at low environmental concentrations, copper shows distinct affinity to the fish liver (Jezierska and Witeska, 2006).
The research observations were exhaustively discussed even in view of limited scientific information on heavy metal bioaccumulation of fresh samples of M. macrobrachion, P. notialis and P. monodon from the study areas (Ologe lagoon, Badagry creek and Lagos lagoon). However, the result obtained in this study has provided substantial scientific information on the subject of heavy metal concentration of these commercially important fish species. The presented results elucidated the importance of shrimps and prawns as an ideal dietetic food due to their overall nutritional benefits. It was observed that high concentrations of some heavy metals were above the WHO permissible limits, making them inimical to human safety. Due to the importance of this study, It can thus be recommended that more scientific research or investigations are needed in future.
References
- Akan, J.C. S Mohmoud, BS Yikala, VO Ogugbuaja (2012): Bioaccumulation of Some Heavy Metals in Fish Samples from River Benue in Vinikilang, Adamawa State, Nigeria, American Journal of Analytical Chemistry. Vol.3 (11): 10.
- Akaahan, T.J., Oluma, H.O.A, Sha'Ata, R. (2010): Physico-chemical and Bacteriological Quality of Water from Shallow Wells in Two Rural Communities in Benue State, Nigeria. Pakistan Journal of Analytical & Environmental Chemistry. Vol 11 (1): 16.
- Alinnor, J., Njoku, P., Ibe, F.C. and Opara, A. (2016) Seasonal variability of carbon monoxide (CO) in the ambient environment of Imo State, Nigeria International Letters of Natural Sciences vol. 53: 40.
- Anani, O.A and Olomukoro, J.O. (2018): Health Risk from the Consumption of Freshwater Prawn and Crab Exposed to Heavy Metals in a Tropical River, Southern Nigeria, Journal of Heavy Metal Toxicity and Diseases vol 3 (25): 1–7.
- Bury, N.R, Walker, P.A and Glover, C.N (2003): Nutritive metal uptake in teleost fish. Journal of experimental biology 206 (1): 11-23.
- Elliot, J.P. and Bergerud (1986): Dynamics of caribou and wolves in northern British Columbia. Canadian Journal of Zoology 1–15
- European Union, (2002): Anti-discrimination law and the European Union Oxford University Press, Oxford studies in European law 234.
- FAO, (2003) Diet, Nutrition, and the Prevention of Chronic Diseases: Report of a Joint board WHO 148.
- Farkas, A. Salánki, J. and A. Specziár (2000): Relation Between Growth and the Heavy Metal Concentration in Organs of Bream Abramis brama L. Populating Lake Balaton. Environmental Contamination and Toxicology 43: 236–243.
- Ganugapenta A. Sreenivasulu, Jayaraju Nadimikeri A, N, Sundara Raja Reddy Balam Chinnapolla B, Lakshmanna Ballari A, Rajasekhar Madiga A, Nirmala, K. C., Lakshmi Prasad Tella D. (2018): Assessment of heavy metal pollution from the sediment of Tupilipalem Coast, southeast coast of India. International Journal of Sediment Research 33: 294 - 302.
- Islam, M.S., Ahmed, M.K., Mamum, M.H. and Hoque, M.F. (2014): Preliminary assessment of heavy metal contamination in surface sediments from a river in Bangladesh Environmental Earth Sciences Vol 10 (7): 1–15
- Jahan, N. Sadiq, N. Zeshan, M. and Tahir, M. (2016): How to Conduct a systemic review: A narrative literature review. Cureus 8(11): e864.
- Kayalar, N., Leibovich, B.C., Orszulak, T.A., et al. (2010) Concomitant Surgery for Renal Neoplasm with Pulmonary Tumor Embolism. The Journal of Thoracic and Cardiovascular Surgery, 139, 320-325.
- Krishna, J.C., Filho, E.A.C. and De Jesus, H.C. (2014). Trace metal contamination in Estuarine Fishes from Vitoria bay, ES, Brazil. Brazilian Archives of Biotechnology 47.5: 765-774.
- Oyeyele, P. (2003): Pollution Assessment Report of the Nairobi River Basin, UNEP. AWN, Nairobi, pp. 106.
- Thomas, J. A. and Mohaideen (2015): Determination of Some Heavy Metals in Fish, Water and sediments from Bay of Bengal. International journal of chemical Environmental Science vol 33: 234 – 241.
- Ubaidullah, M., & Abdullah, S. (2009). Assessment of heavy metals in sediments of the river Ravi, Pakistan. Int. J. Agric. Biol, 197–200.
- World Health Organization (2003), “Global Environment Monitoring System/Water Operational Guide,” World Health Organization, Geneva.
- Won,D.*† Su-Ning Zhu,*† Mian Chen,*† Anouk-Martine Teichert,‡ Jason E. Fish,* Charles C. Matouk,§ Michael Bonert,§ Matadial Ojha,¶ Philip A. Marsden,*§ and Myron I. Cybulsky*† (2007): Relative Reduction of Endothelial Nitric-Oxide Synthase Expression and Transcription in Atherosclerosis-Prone Regions of the Mouse Aorta and in an in Vitro Model of Disturbed Flow. Am J Pathol. 171(5): 1691–1704.
Citation: Adeboyejo O. Akintade, Clarke, O. Edwin and Badmos Lateef. (2023). Assessment of Heavy metals in Shrimps; Penaeus notialis, Penaeus Monodon, and Prawn; Macrobrachium Macrobrachion Sampled from three [3] water Bodies in Lagos State, Nigeria: An Inimical to Human Health Safety Issues. Journal of Agriculture and Aquaculture 5(3).
Copyright: © 2023 Adeboyejo O. Akintade. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.