Research Article
Volume 7 Issue 1 - 2025
Selection of Probiotic Bacteria from some Bacillus Species Isolated from the Skin and Gastrointestinal Tract of Catfish (Clarias Anguillaris) in Makurdi Metropolitan, Nigeria.
1Department of Animal Health and Production, College of Veterinary Medicine, Joseph Sarwuan Tarka University, Makurdi, Benue State, Nigeria
2Department of Veterinary Microbiology, College of Veterinary Medicine, Joseph Sarwuan Tarka University, Makurdi, Benue State, Nigeria
2Department of Veterinary Microbiology, College of Veterinary Medicine, Joseph Sarwuan Tarka University, Makurdi, Benue State, Nigeria
*Corresponding Author: Escientific Publishers, Department of Animal Health and Production, College of Veterinary Medicine, Joseph Sarwuan Tarka University, Makurdi, Benue State, Nigeria.
Received: February 07, 2025; Published: February 19, 2025
Abstract
Thirteen Bacillus species including Bsp1-Bsp13 were isolated and screened for probiotic properties. Apparently healthy fish samples of Clarias anguillaris with mean weight and total length of 96 ± 28 grams and 24±22 centimeters were obtained randomly from Federal University of Agriculture, Makurdi (FUAM) Fish Hatchery Complex where the research was conducted. These Bacillus species isolates were subjected to mucosal attachment (colonization) test for adhesive ability, gastrointestinal tract (stomach, duodenum, intestine) transit and thereafter pathogenicity test. The results revealed that isolates Bsp13 (OD620 0.044) adhered significantly (P ≤ 0.05) better to the mucus than all the other isolates with least adhesion recorded with Bsp6 (OD620 0.001) which shows a better potential to survive and proliferate in the GIT environment and effectively to discharge the desired probiotic effects. The survival of Bacillus species in gastrointestinal juice (25mM Nacl, 7mM Kcl, 45mM NaHCO3, 0.3% Pepsin) revealed all isolates thrived at varying degrees along the GIT transit except Bsp11. Bacillus species Bsp2, Bsp3, Bsp9, Bsp10 and Bsp12 were resistant to the simulated duodenal juice DJ (1%w/v bovine bile) and intestinal juice IJ (0.3% bovine bile and pancreatin) due to their increase in population compared to the initial. In the course of GIT transit, Bsp9, Bsp10, Bsp11, and Bsp13 were found worthy to be chosen due to their performance in the simulated GJ, DJ, and finally IJ. The 90-100% survival rate recorded and good growth performance with significant difference suggests that the isolates selected in this study are safe for use in the catfish and for growth promotions. So, in this study, Bsp3, Bsp5, Bsp9, Bsp10 and Bsp13 showed good initial concentration of above 8LogCFUg-1 and after activation, passed through the simulated GIT and maintained the concentration above 8LogCFUg-1. The aim of the work was to select and evaluate potential probiotic bacteria among some Bacillus species isolated from the skin and intestine of indigenous catfish.
Keywords: Probiotic bacteria; Selection; Skin; Gastrointestinal tract; Indigenous; Catfish
Introduction
When looking at probiotics intended for aquatic usage, it is important to consider certain influencing factors that are fundamentally different from terrestrial based probiotics. Aquatic animals have a much closer relationship with their external environment which is water. Potential pathogens are able to maintain themselves in the same external environment of the animal (water) and proliferate independently of host animal (Monica and Jayaraj, 2021). In selecting probiotics, microorganisms with beneficial health effects for the host, many criteria have to be met. However, it is important to know the mode of actions used by bacteria to achieve the beneficial effect to the host. The mechanisms used include, the production of inhibitory substances against pathogens, competition for essential nutrients and adhesion sites, the supply of essential nutrients and enzymes resulting in enhanced nutrition in the host and the modulation of interaction with the environment and the development of beneficial immune response (Oyetayo and Oyetayo, 2005; Sihang and Sharma, 2012). Oyetayo and Oyetayo (2005) reported some properties that a good probiotic must possess including; not to be harmful to the host, acceptable to the host, reach the action site where the effect is required, work in-vivo and in-vitro and should not possess virulence gene, or antibiotic resistance genes like plasmids. A successful probiotic is expected to have a few specific properties in order to certify a beneficial effect.
A common way to select probiotics is to perform in-vitro antagonism tests, in which pathogens are exposed to the candidate probiotics or their extracellular products. The steps to select probiotic bacteria for use in aquaculture according to Gomez-Gil et al.(2000) involves: collection of background information; acquisition of potential probiotics ordinarily from the host of its intended target, evaluation of the ability of potential probiotics to out-compete pathogenic strains; assessment of the pathogenicity of the potential probiotics on the target species; evaluation of the performance of the potential probiotics in the targeted host; and finally its economic cost/benefit analysis. The ability of microorganisms to colonize is often considered as one of the main selection criteria for potential probiotics, that is, the efficient adherence to intestinal epithelial cells to reduce or prevent colonization of pathogens (Vine et al., 2004). In addition, potential probiotics must exert its beneficial effects (e.g. enhanced nutrition and increased immune response) in the host (Vine, 2004). Finally, the probiotic must be viable under normal storage conditions and also suitable for industrial processes (Sahadeva et al., 2011).
Bacillus species is a genus of Gram positive, rod shaped, aerobic or facultative anaerobic and spore forming bacteria that is wide spread in the environment. The genus is composed of approximately 77 different species (Ngau and Phu, 2011). Bacillus species have a long history of use in biotechnology and as dietary supplements for humans and animals (Ezema,2013) and aquaculture (Ashiraf et al., 2013) of agricultural importance. Bacillus species are ubiquitous in nature, but are found in higher concentration in soil, water, and food products that have plant origin. Bacillus bacteria form endospore under stressful conditions which protect the dormant bacteria inside for years and can be resistant to extreme heat, radiation, freezing, drying, and chemical disinfectants (Christian-Teodor et al., 2014) and when conditions are favorable for growth, the endospore converts to a vegetative cell which can thrive again. This makes Bacillus suitable for use, even in harsh areas especially aquatic environment (Ashiraf et al., 2013).
The ability to form spores is beneficial and allows for long?term storage without the loss of viability compared to those species that are non-spore?forming bacterium. Also, spores are able to survive the harsh, low pH of the gastric barrier and can reach the small intestine to exert their probiotic properties (Cutting, 2011). Strains of Bacillus are very good potential candidates to be used as probiotics (Schaltz et al., 2017). Mulalo et al. (2014) summarized some of the probiotic attribute of Bacillus spp including gastrointestinal tract stress tolerance, good adhesion capability, having bio-therapeutic properties, safe transit and localization in the gut, good survival life within animal GIT, sporulate and should be active even in water environment.
A distinctive feature of Bacillus species is high proteolytic activities which play roles in activation of regeneration process, the enhancement or normal digestion and degeneration of allergic component. In in vitro trials, the tolerance of Bacillus spp to acid and bile salts reflect their survival rate and ability through gastrointestinal tract. (Christian-Teodor et al., 2014). Their heat stability and ability to survive the gastric barrier makes them attractive as food supplements and this use is now growing (Cutting, 2016).
Bacillus bacteria produce many kinds of metabolites which could affect the other microbes in the fish gut. The bacteriocin is antimicrobial substances which are bactericidal or bacteriostatic peptide that are mostly active against pathogenic bacteria. In order to be able to stimulate the gut immune system, probiotic bacteria should be resistant to the enzymes in the oral cavity (amylase and lysozyme), to low pH in the stomach and to the concentration of bile, pancreatic juice and mucus in the small intestine which are important for survival and successful transit through the gastrointestinal tract. Microorganisms as probiotic must also be able to persist within and adhere to the gut epithelial tissues to stimulate the phagocytic cells as have been reported for Bacillus species by Chauhan and Singh (2019). Bacillus species have been included in various supplements for human, terrestrial and aquatic animals to protect against diseases, increase immunity, improves growth performance and water quality to favor the culture organisms used in many countries of the world (Fouad et al., 2017).
Different Bacillus strain has been reported to display antimicrobial, anti-oxidative and immune-modulatory activity in the host and has been shown to possess pathogen exclusion and food fermentation ability (Ripert et al., 2016). Some strains of Bacillus species produce metabolites to accomplish growth performances (Quattara et al., 2017; Takana, 2016). All these metabolites possessed by Bacillus species qualify them serve as good probiotic bacteria for use in aquaculture
Materials and Methods
Experimental fish
Apparently healthy fish samples of Clarias anguillaris of both sexes with mean weight and total length of 96 ± 28 grams and 24 ± 22 centimeters respectively were obtained randomly from Federal University of Agriculture, Makurdi (FUAM) Fish Hatchery Complex and 6 homestead fish ponds in Makurdi Metropolitan at various times. The fishes were caught randomly from either concrete or earthen ponds using dragging net measuring 6x2m2 with mesh size of 2cm2 by two trained fishermen. The fish species were identified with the aid of pictorial fish diagrams as compiled by Olaosebikan and Raji (2004). The fishes obtained were transported live to the veterinary microbiology laboratory Federal University of Agriculture, Makurdi in plastic buckets or in 25-liter Jerry-can containing water from the respective sources of the fish. From the laboratory, the culture materials were obtained and subjected to isolation and identification following a standard procedure according to the Gram-positive identification flow chart according to Bergey’s manual. Thirteen Bacillus species were isolated from both skin and gastrointestinal tract of catfish Clarias anguillaris and screened for probiotic properties. The objective of this experiment is to select most successful Bacillus species with good probiotic properties that can be used for catfish production. There are various methods of selection, but this research adopted selection criterion described by Rini et al. (2014) following screening processes performed.
Apparently healthy fish samples of Clarias anguillaris of both sexes with mean weight and total length of 96 ± 28 grams and 24 ± 22 centimeters respectively were obtained randomly from Federal University of Agriculture, Makurdi (FUAM) Fish Hatchery Complex and 6 homestead fish ponds in Makurdi Metropolitan at various times. The fishes were caught randomly from either concrete or earthen ponds using dragging net measuring 6x2m2 with mesh size of 2cm2 by two trained fishermen. The fish species were identified with the aid of pictorial fish diagrams as compiled by Olaosebikan and Raji (2004). The fishes obtained were transported live to the veterinary microbiology laboratory Federal University of Agriculture, Makurdi in plastic buckets or in 25-liter Jerry-can containing water from the respective sources of the fish. From the laboratory, the culture materials were obtained and subjected to isolation and identification following a standard procedure according to the Gram-positive identification flow chart according to Bergey’s manual. Thirteen Bacillus species were isolated from both skin and gastrointestinal tract of catfish Clarias anguillaris and screened for probiotic properties. The objective of this experiment is to select most successful Bacillus species with good probiotic properties that can be used for catfish production. There are various methods of selection, but this research adopted selection criterion described by Rini et al. (2014) following screening processes performed.
Mucosal Attachment (colonization) test of Bacillus species
The ability of bacteria to colonize the gut and adhere to the epithelial surface and consequently interfere with the adhesion of pathogens is a desirable criterion in the selection of probiotics (Lazado et al., 2012). The experiment was conducted in bacterial research laboratory NVRI Vom, Nigeria. Fifty adult Clarias anguillaris were purchased in a homestead pond in Jos town and was transported to the Laboratory in a 50-liter round-bottom rubber basin and aloud for 48 hours to stabilized and were not fed.
The ability of bacteria to colonize the gut and adhere to the epithelial surface and consequently interfere with the adhesion of pathogens is a desirable criterion in the selection of probiotics (Lazado et al., 2012). The experiment was conducted in bacterial research laboratory NVRI Vom, Nigeria. Fifty adult Clarias anguillaris were purchased in a homestead pond in Jos town and was transported to the Laboratory in a 50-liter round-bottom rubber basin and aloud for 48 hours to stabilized and were not fed.
Isolation and Processing of Crude Mucus from Intestinal Mucosa
The protocol of Ronan et al., (1999) and Balakrishna (2013) were employed for collection and processing the crude mucus. The fish were fasted for 48 hours in the container and the water was changed twice a day (morning and evening). The fish were sacrificed humanely one after the other by giving a shock of blow on the head. The abdominal region of each fish was incised with a scalpel bled to expose the abdominal content. The intestine was severed out and placed into a sterile petri dish. The intestine was incised longitudinally with different scalpel blade to expose the intestinal lumen. With the use of spatula, mucus was scrapped from the luminal surface into a sterile petri-dish. The mucus was mixed with normal saline at the ratio of 1:4 using automatic pipette. The mixture was homogenized using vortex (Maxi mix II, thermolyne, USA) to obtain uniform mixture. The mixture was distributed into Eppendorf tubes and centrifuged at high speed of 14,000 rpm (24xg) for 15 minutes using Eppendorf Centrifuge 5417®, Germany. The supernatant was collected into another new Eppendorf tube and centrifuged for the second time at the same speed. Thereafter the supernatant was sterilized under UV light for 30 minutes inside biosafety cabinet class II (SteriGARD® USA). The mucus solution was stored at -20°C until use.
The protocol of Ronan et al., (1999) and Balakrishna (2013) were employed for collection and processing the crude mucus. The fish were fasted for 48 hours in the container and the water was changed twice a day (morning and evening). The fish were sacrificed humanely one after the other by giving a shock of blow on the head. The abdominal region of each fish was incised with a scalpel bled to expose the abdominal content. The intestine was severed out and placed into a sterile petri dish. The intestine was incised longitudinally with different scalpel blade to expose the intestinal lumen. With the use of spatula, mucus was scrapped from the luminal surface into a sterile petri-dish. The mucus was mixed with normal saline at the ratio of 1:4 using automatic pipette. The mixture was homogenized using vortex (Maxi mix II, thermolyne, USA) to obtain uniform mixture. The mixture was distributed into Eppendorf tubes and centrifuged at high speed of 14,000 rpm (24xg) for 15 minutes using Eppendorf Centrifuge 5417®, Germany. The supernatant was collected into another new Eppendorf tube and centrifuged for the second time at the same speed. Thereafter the supernatant was sterilized under UV light for 30 minutes inside biosafety cabinet class II (SteriGARD® USA). The mucus solution was stored at -20°C until use.
Mucosal attachment test was a determination of the adhesive ability of Bacillus strains:
The Crystal Violet method was used to determine the adhesive ability of the selected strains according to method described by Vesterlund et al. (2005). The supernatant was removed from the freezer at -20°C and allowed to be thawed. Then 150µL of mucus were dispensed into 96 micro-titer wells using multi-micropipette (Multi talt finnpipette Lab system, Finland). These were incubated overnight (18-24hrs) at 4oC. The micro-titer wells were washed 2 times with phosphate buffer saline (PBS) and was dry bloated before use again. One hundred microliters (100µL) of the test isolate were then added into the wells which had already been coated with the mucus leaving one raw of the micro-titer wells as control wells and incubated for 90 minutes at 37OC inside ELISA shaker/incubator (Heidolph Titramax 1600, Germany). Thereafter, the cells were fixed at 60°C for 20 minutes and 100 µL/well of 0.1% of crystal violet was added and incubated for 30 minutes at same temperature. The wells were washed 5 times with PBS and the plate was bloat-dried again. To release the fixed and attached cells to mucus, 100µL of Citrate Buffer (24.087g sodium citrate dehydrate, 3.471g citric acid, pH 4.3) was added to each well and incubated for 45 minutes at room temperature. The micro-titer plate was then read in a micro-titer plate reader (Multiskan Ex Thermoscientific, USA). Results were expressed by subtracting the absorbance value of the control from the absorbance value recorded for the samples according to Vesterlund et al. (2005). Each experiment was done in triplicate.
The Crystal Violet method was used to determine the adhesive ability of the selected strains according to method described by Vesterlund et al. (2005). The supernatant was removed from the freezer at -20°C and allowed to be thawed. Then 150µL of mucus were dispensed into 96 micro-titer wells using multi-micropipette (Multi talt finnpipette Lab system, Finland). These were incubated overnight (18-24hrs) at 4oC. The micro-titer wells were washed 2 times with phosphate buffer saline (PBS) and was dry bloated before use again. One hundred microliters (100µL) of the test isolate were then added into the wells which had already been coated with the mucus leaving one raw of the micro-titer wells as control wells and incubated for 90 minutes at 37OC inside ELISA shaker/incubator (Heidolph Titramax 1600, Germany). Thereafter, the cells were fixed at 60°C for 20 minutes and 100 µL/well of 0.1% of crystal violet was added and incubated for 30 minutes at same temperature. The wells were washed 5 times with PBS and the plate was bloat-dried again. To release the fixed and attached cells to mucus, 100µL of Citrate Buffer (24.087g sodium citrate dehydrate, 3.471g citric acid, pH 4.3) was added to each well and incubated for 45 minutes at room temperature. The micro-titer plate was then read in a micro-titer plate reader (Multiskan Ex Thermoscientific, USA). Results were expressed by subtracting the absorbance value of the control from the absorbance value recorded for the samples according to Vesterlund et al. (2005). Each experiment was done in triplicate.
Gastrointestinal transit survival test
The objective of gastrointestinal survival test was to ascertain the probiotic bacteria to emerge is capable of passing through the gastrointestinal tract (gastric lumen, duodenum and the intestine) to reach alive the intestine which is the site of action of the probiotics. The experiment was done at National Veterinary Research Institute (NVRI), Vom, Plateau State. The modified method of Ayeni et al. (2011) was employed for the gastrointestinal transit survival test using chemically simulated techniques for the successful isolates.
The objective of gastrointestinal survival test was to ascertain the probiotic bacteria to emerge is capable of passing through the gastrointestinal tract (gastric lumen, duodenum and the intestine) to reach alive the intestine which is the site of action of the probiotics. The experiment was done at National Veterinary Research Institute (NVRI), Vom, Plateau State. The modified method of Ayeni et al. (2011) was employed for the gastrointestinal transit survival test using chemically simulated techniques for the successful isolates.
Few colonies of overnight culture of test isolate on MRS (TM Media, TM 146-India) agar was transferred into MRS (TM Media, TM 147-India) broth and incubated for 48hrs at 37°C. Serial dilution was made after the incubation and plated on MRS agar followed by further incubation for 48 hours at 37°C. The remaining broth solution was centrifuged at 7000 rpm for 15 minutes using high speed centrifuge (Hattrich Universal, Germany). The supernatant was discarded and the sediment was washed two times with sterile physiological buffer saline (PBS). The sediment was re-suspended in the solution of simulated gastrointestinal juice (25mM NaCl, 7 mM KCl, 45mM NaHCO3 and 0.3% pepsin) at different pH of 1.3, 3.0, 1.3+skimmed milk in triplicates. This was incubated for 90 minutes at 37°C; thereafter serial dilutions were performed plated on MRS agar and incubated for 48 hrs. At same temperature.
Duodenal transit
For the duodenal transit test, the suspension was centrifuged again at 7000 rpm for 15 minutes using same centrifuge, and the sediment was re-suspended in the simulated duodenal juice (1% bovine bile, pH 8), then was incubated for 10 minutes at 37°C in anaerobic condition (10% H2, 10% CO2, 80% N2). Serial dilution fold of 10 dilution was made and plated on MRS agar incubated for 48hrs. At 37°C.
For the duodenal transit test, the suspension was centrifuged again at 7000 rpm for 15 minutes using same centrifuge, and the sediment was re-suspended in the simulated duodenal juice (1% bovine bile, pH 8), then was incubated for 10 minutes at 37°C in anaerobic condition (10% H2, 10% CO2, 80% N2). Serial dilution fold of 10 dilution was made and plated on MRS agar incubated for 48hrs. At 37°C.
Intestinal transit
For the intestinal transit test, the isolate suspension from the previous duodenal test was centrifuged at 7000 rpm for 15 minutes and the sediment was re-suspended in the simulated intestinal juice (0.3% bile, 0.1% porcine pancreatin, pH 8) which was incubated for 180 minutes under anaerobic condition (10% H2, 10% CO2 and 80% N2). After the incubation, serial dilution was finally done and plated on MRS agar followed by incubation for 48hrs. For viable count. All serial dilution was done in triplicate. Plate counts were done using manual tally counter and plates with colony count of 30-300 were considered for evaluation. The final percentage of survival was calculated from the difference of recovered bacteria after complete GIT challenge and CFU of initial bacteria.
For the intestinal transit test, the isolate suspension from the previous duodenal test was centrifuged at 7000 rpm for 15 minutes and the sediment was re-suspended in the simulated intestinal juice (0.3% bile, 0.1% porcine pancreatin, pH 8) which was incubated for 180 minutes under anaerobic condition (10% H2, 10% CO2 and 80% N2). After the incubation, serial dilution was finally done and plated on MRS agar followed by incubation for 48hrs. For viable count. All serial dilution was done in triplicate. Plate counts were done using manual tally counter and plates with colony count of 30-300 were considered for evaluation. The final percentage of survival was calculated from the difference of recovered bacteria after complete GIT challenge and CFU of initial bacteria.
Pathogenicity test of Bacillus species on catfish
The objective of this was to ascertain the safety of the successful potential probiotic bacteria to the live target host. A complete randomized design was employed in this test. One hundred and twenty (120) Clarias anguillaris juveniles obtained from homestead fish farmer in Makurdi metropolitan and transported to experimental site. After stabilizing the fish for 2 weeks they were distributed into 8 groups comprising 10 fish each and in replicate. The pathogenicity test was carried out according to the modified protocol of Edward et al. (2010) and Sarka and Rashid (2012). All the 10-target fish in positive control group were injected intramuscularly with 0.2mL of potential fish pathogen Vibrio alginolytius at the concentration of 108 CFU/mL that was estimated with 0.5 McFarland standards. All the fish in negative control group were injected intramuscularly with 0.2 mL PBS. All fish in test groups were injected intramuscularly with 0.2mL of test Bacillus species at the concentration of 108 CFU/mL-1. The test groups were according to the number of successful Bacillus species selected through the screenings.
The objective of this was to ascertain the safety of the successful potential probiotic bacteria to the live target host. A complete randomized design was employed in this test. One hundred and twenty (120) Clarias anguillaris juveniles obtained from homestead fish farmer in Makurdi metropolitan and transported to experimental site. After stabilizing the fish for 2 weeks they were distributed into 8 groups comprising 10 fish each and in replicate. The pathogenicity test was carried out according to the modified protocol of Edward et al. (2010) and Sarka and Rashid (2012). All the 10-target fish in positive control group were injected intramuscularly with 0.2mL of potential fish pathogen Vibrio alginolytius at the concentration of 108 CFU/mL that was estimated with 0.5 McFarland standards. All the fish in negative control group were injected intramuscularly with 0.2 mL PBS. All fish in test groups were injected intramuscularly with 0.2mL of test Bacillus species at the concentration of 108 CFU/mL-1. The test groups were according to the number of successful Bacillus species selected through the screenings.
Results
Mucosal attachment tests of Bacillus species
The mucosal attachment test revealed differences in adhesion among the 13 isolates. The attachment pattern of the thirteen Bacillus species is presented on figure 1. All selected isolates competed and adhered at varying degrees with few showing poor adhesion to the intestinal mucus. Among the selected isolates, Bsp13 (OD620 0.044) adhered significantly (P ≤ 0.05) better than all the other isolates tested. Other isolates followed according to the degree of adhesion to mucus of the intestine of fish include: Bsp1 (OD620 0.034), Bsp12 (OD620 0.030), Bsp3 (OD620 0.029) Bsp10 (OD620 0.028) and Bsp11 (OD620 0.028. The weakest in capacity of adhesion were Bsp6 (OD620 0.001), followed by Bsp5 (OD620 0.009), Bsp4 (OD620 0.014) and Bsp9 (OD620 0.018).
The mucosal attachment test revealed differences in adhesion among the 13 isolates. The attachment pattern of the thirteen Bacillus species is presented on figure 1. All selected isolates competed and adhered at varying degrees with few showing poor adhesion to the intestinal mucus. Among the selected isolates, Bsp13 (OD620 0.044) adhered significantly (P ≤ 0.05) better than all the other isolates tested. Other isolates followed according to the degree of adhesion to mucus of the intestine of fish include: Bsp1 (OD620 0.034), Bsp12 (OD620 0.030), Bsp3 (OD620 0.029) Bsp10 (OD620 0.028) and Bsp11 (OD620 0.028. The weakest in capacity of adhesion were Bsp6 (OD620 0.001), followed by Bsp5 (OD620 0.009), Bsp4 (OD620 0.014) and Bsp9 (OD620 0.018).
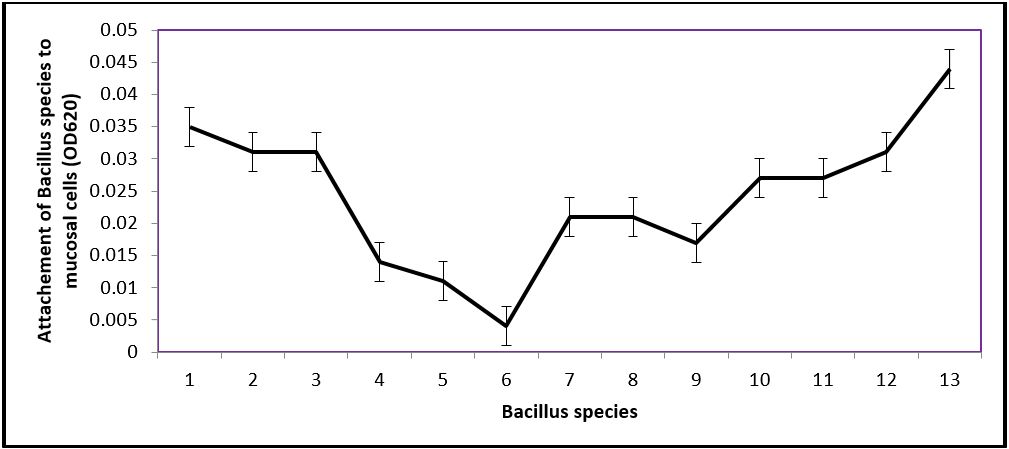
Figure 1: Absorbance curve of Bacillus species observed in attachment ability in gut of fish (C. anguillaris).
Among these isolates subjected to mucosal attachment tests, Bsp1, Bsp3, Bsp10, Bsp11 and Bsp12 with Bsp13 demonstrated a good attachment capability recording significantly highest values (0.150 ± 0.01) and mean difference of OD6200.044. All Bacillus species with significant difference (P ≤ 0.05) from the control have better chance of attachment (Table 1).
| Bacillus species | Optic density (OD620 ± SD) | Mean – control (OD620, 0.106) |
| Bsp1 | 0.141±0.02ab | 0.035 |
| Bsp2 | 0.137±0.01ab | 0.031 |
| Bsp3 | 0.137±0.07ab | 0.031 |
| Bsp4 | 0.120±0.02bc | 0.014 |
| Bsp5 | 0.117±0.02bc | 0.011 |
| Bsp6 | 0.110±0.10c | 0.004 |
| Bsp7 | 0.127±0.01abc | 0.021 |
| Bsp8 | 0.127±0.11abc | 0.021 |
| Bsp9 | 0.123±0.02bc | 0.017 |
| Bsp10 | 0.133±0.01abc | 0.027 |
| Bsp11 | 0.133±0.02abc | 0.027 |
| Bsp12 | 0.137±0.01ab | 0.031 |
| Bsp13 | 0.150±0.01a | 0.044 |
abcmean with different superscript within the same column differ significantly (P≤0.05)
Table 1: Attachment capability of Bacillus species isolated and identified in the scrapped intestinal mucosa of C. anguilaris.
Table 1: Attachment capability of Bacillus species isolated and identified in the scrapped intestinal mucosa of C. anguilaris.
Gastrointestinal survival transit in simulated gastric, duodenal and intestinal juices
Thirteen Bacillus species were subjected to GIT transit test; only 10 responded and were featured in this experiment. The survivability pattern of the 10 Bacillus species under selection that journeyed the different steps of simulated GIT is presented on figure 2(a-f) and figure 3(g-j) below.
Thirteen Bacillus species were subjected to GIT transit test; only 10 responded and were featured in this experiment. The survivability pattern of the 10 Bacillus species under selection that journeyed the different steps of simulated GIT is presented on figure 2(a-f) and figure 3(g-j) below.
Survival of Bacillus species in Gastrointestinal Juice (25mM Nacl, 7mM Kcl, 45mM NaHCO3, 0.3% Pepsin)
Bacillus species tested thrived at varying degrees along the GIT transit except Bsp11. In all cases the addition of 10% skimmed milk to GJ pH 1.3 which raised the pH close to pH 5.0 or the use of pH 3.0 increased the viability and the differences in simulated DJ (1% bovine bile, pH 8) became apparent. Bacillus species Bsp1, Bsp4, Bsp5, Bsp11 and Bsp13 (figure 2 a, d, e, 3h and j), were all sensitive to the simulated gastric juice in pH 1.3, 3.0 and 1.3+10% skimmed milk, which was evident due to decrease in population compared to the initial colony forming unit. While Bsp2, Bsp3, Bsp9, Bsp10 and Bsp12 (figure 2 b, c, f, 3g and i) were resistant due to their increase in population compared to the initial. The multiplication of Bsp10 in pH 1.3 + skimmed milk was glaringly apparent and the multiplication of Bsp7 pH 3.0 in GJ was visibly pronounced. For Bsp4, despite being sensitive, the growth in pH1.3 was higher than the other pH levels (figure 2d). The growth of Bsp1 in GJ was at the same frequency in all pH level.
Survival of Bacillus species in Duodenal Juice (1%w/v bovine bile)
After the adverse effects of the GJ, the sensitivity and resistance to the duodenal juice differ among the 10 Bacillus species observed in different pH levels. Bsp1, Bsp4, Bsp10 and Bsp12 (figure 2 a, d, and figure 3g and i) resisted better in the high bile salt concentration showing higher viability increase than others that were apparently above 8logCFUmL-1. However, almost all were resistant to the DJ which resulted in increased more than those in the GJ. The multiplication of Bsp1, Bsp3, Bsp9, were steady at same level in all pH 1.3+10% skimmed milk. The resistance of Bsp4, Bsp9, Bsp10 and Bsp12, were commendable in the DJ (figure 2 d, f, figure 3 g and i).
After the adverse effects of the GJ, the sensitivity and resistance to the duodenal juice differ among the 10 Bacillus species observed in different pH levels. Bsp1, Bsp4, Bsp10 and Bsp12 (figure 2 a, d, and figure 3g and i) resisted better in the high bile salt concentration showing higher viability increase than others that were apparently above 8logCFUmL-1. However, almost all were resistant to the DJ which resulted in increased more than those in the GJ. The multiplication of Bsp1, Bsp3, Bsp9, were steady at same level in all pH 1.3+10% skimmed milk. The resistance of Bsp4, Bsp9, Bsp10 and Bsp12, were commendable in the DJ (figure 2 d, f, figure 3 g and i).
Survival of Bacillus species in Intestinal Juice (0.3% bovine bile and pancreatin)
The final journey to the IJ did not affect the viability instead there was significant increase in the multiplication in almost all except for Bsp2, Bsp4, Bsp9 and Bsp11 (fig. 2 b, d, f, fig. 3 h). Bsp1 population decreased in the GJ but increased when entered the DJ and IJ. The acidic level of GJ did not affect Bsp2 with evidence of increase when the bacteria entered GJ, but decreased when in DJ, however, those Bacillus species in pH 1.3 maintained their course through the journey. The growth of Bsp3, Bsp4 and Bsp5 fluctuated, but maintained similar population as the initial before reaching the IJ. Bsp12, showed a steady increase from initial population to the GJ, but multiplied greatly in the DJ and IJ. Bsp13 was sensitive to GJ and DJ, but exceedingly multiplied above the initial population when the bacteria reached the IJ. The growth of Bsp9 was maintained uniformly across the journey from GJ to IJ. However, Bsp10 exceedingly multiplied sharply from the initial to GJ and reached highest population in the IJ. The Bsp11 recorded the poorest performance in the journey with evidence of serious dropping far below the initial population.
The final journey to the IJ did not affect the viability instead there was significant increase in the multiplication in almost all except for Bsp2, Bsp4, Bsp9 and Bsp11 (fig. 2 b, d, f, fig. 3 h). Bsp1 population decreased in the GJ but increased when entered the DJ and IJ. The acidic level of GJ did not affect Bsp2 with evidence of increase when the bacteria entered GJ, but decreased when in DJ, however, those Bacillus species in pH 1.3 maintained their course through the journey. The growth of Bsp3, Bsp4 and Bsp5 fluctuated, but maintained similar population as the initial before reaching the IJ. Bsp12, showed a steady increase from initial population to the GJ, but multiplied greatly in the DJ and IJ. Bsp13 was sensitive to GJ and DJ, but exceedingly multiplied above the initial population when the bacteria reached the IJ. The growth of Bsp9 was maintained uniformly across the journey from GJ to IJ. However, Bsp10 exceedingly multiplied sharply from the initial to GJ and reached highest population in the IJ. The Bsp11 recorded the poorest performance in the journey with evidence of serious dropping far below the initial population.
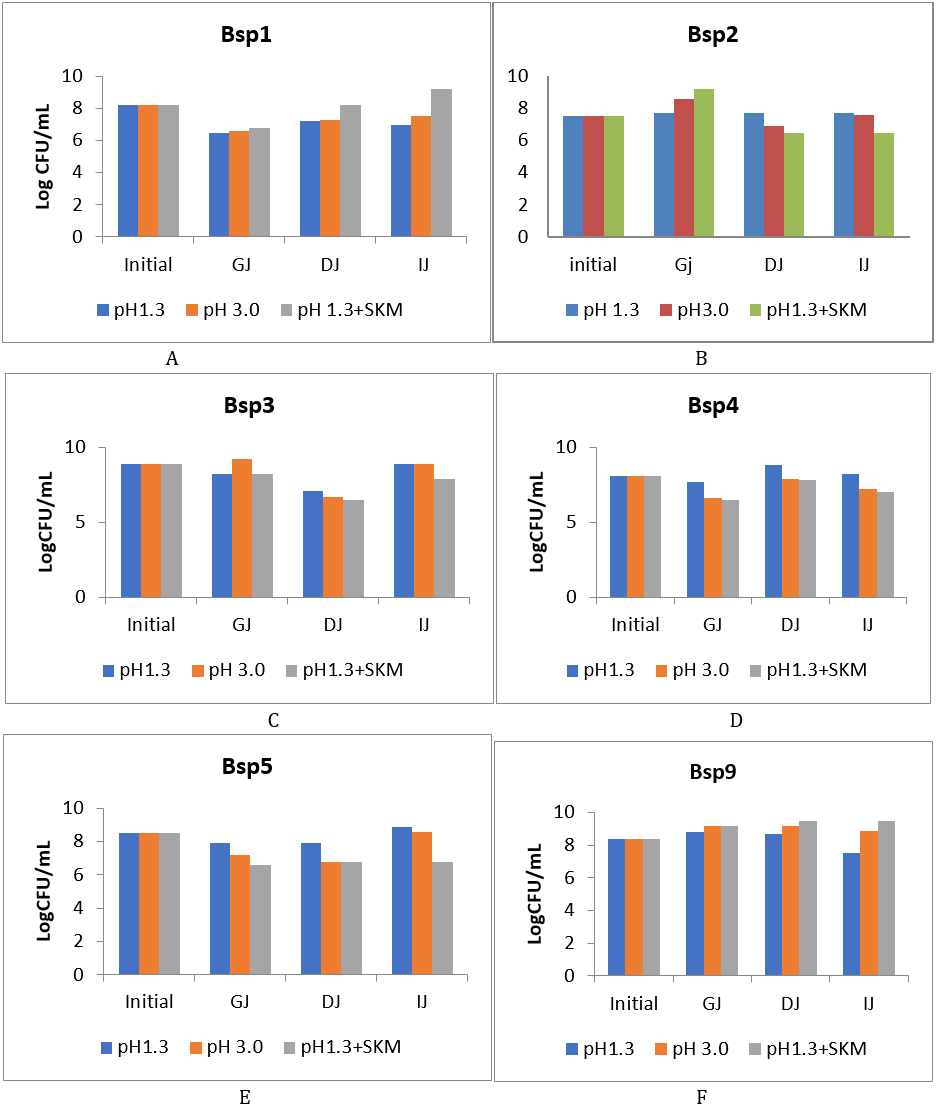
Figure 2 (a-f): Log CFU/ml of isolates of Bacillus species after the chemically simulated gastrointestinal transit. GJ: gastric juice, DJ: duodenal juice and IJ: intestinal juice, SKM = skimmed milk.
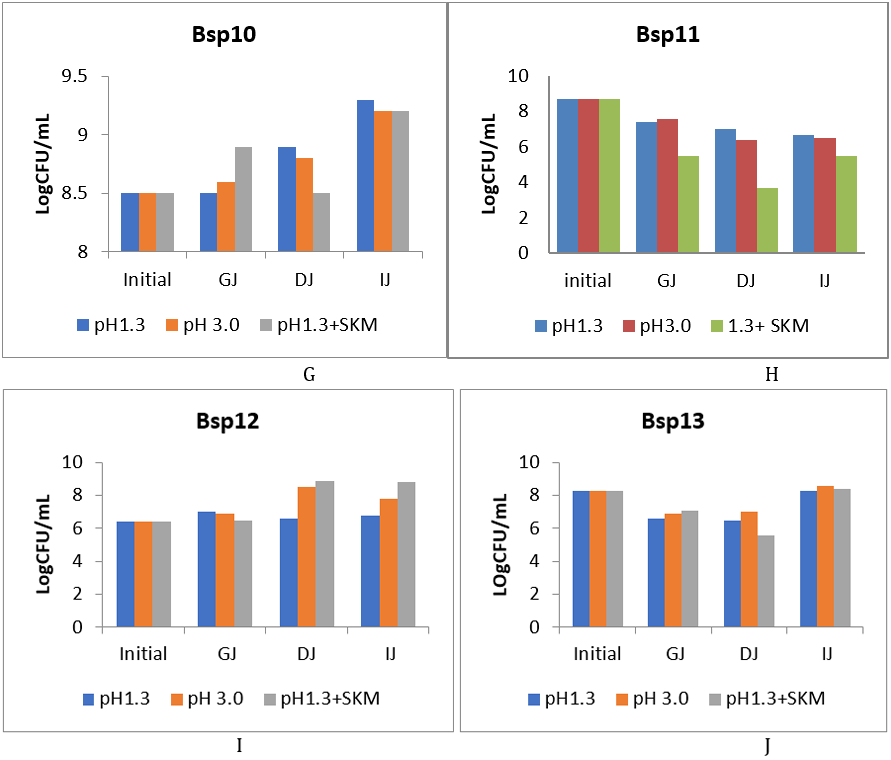
Figure 2 (g-j): Log CFU/ml of isolates of Bacillus species after the chemically simulated gastrointestinal transit. GJ: gastric juice, DJ: duodenal juice and IJ: intestinal juice, SKM = skimmed milk.
With regard to individual performance in the GIT transit, Bsp1 can reach the IJ in good population only when supplemented with 10% skimmed milk having 9LogCFU-1. Bsp2 increased significantly higher in GJ especially when supplemented with 10% skimmed milk and maintained the level with initial in DJ and IJ but did not thrive well compared to those in pH 1.3 with skimmed milk and pH 3.0. Bsp3 in pH1.3 maintain its population from the GJ to IJ, but despite dropping in DJ to 7 Log/ml, multiplied rapidly to reach 8LogCFUmL-1 in IJ which differed significantly (P≤0.05) (figure 2c). For Bsp4 and Bsp5 the population in in pH 1.3 was encouraging through the GIT transit, with the growth in pH1.3+skimmed milk reduced between the initial level (figure 2d and e).
The increase of Bsp9 along the transit resulted in more numbers than the initial population with no significant difference which will do well only when pH 1.3 was supplemented 10% skimmed milk recording the highest level (fig. 3h) reaching close to 9LogCFU/ml. The growth of Bsp10 along the transit test at varying degrees recording significant level when pH1.3 supplemented with 10% skimmed milk and very good multiplication was observed with those in pH 1.3 and 3.0 when reaching DJ and multiplied significantly to reach above 9LogCFUmL-1 when arriving the IJ. The sensitivity of Bsp11, recorded the poorest performance compared to the initial level which reduced significantly down to 6LogCFUmL-1 with pH1.3+skimmed milk recording the least growth. Bsp12 recorded the best performance along the transit in all pH levels (fig. 4.5i) with significant increase in DJ and IJ with population reaching the IJ higher than the initial one of 6LogCFUmL-1 although, there was no significant difference (P≥0.05). Similarly, Bsp13b showed sensitivity to the GJ and DJ which decrease down to 6LogCFUmL-1 far lower than 8logCFUmL-1 of the initial population, but the population increased in IJ reaching above 8LogCFUmL-1 with highly significant difference (P ≤ 0.05) in the multiplication.
So, in the course of this GIT transit Bsp9, Bsp10, Bsp11, and Bsp13 were found worthy to be chosen due to their performance in the simulated GJ, DJ, and finally IJ. These isolates have potential to withstand the adverse effect of the GIT transit and reach the target site of action alive and in good population.
Pathogenicity Assay of the Bacillus species on live C. anguillaris juveniles.
This experiment was to assess if the Bacillus species intended to be used as probiotics would be harmful to the target host and as potential of growth promoters. The results of pathogenicity assay of the potential probiotic Bacillus species are presented in the Table 2 below:
This experiment was to assess if the Bacillus species intended to be used as probiotics would be harmful to the target host and as potential of growth promoters. The results of pathogenicity assay of the potential probiotic Bacillus species are presented in the Table 2 below:
| Treatment | Survivability (%) (n=10) | Initial weight(g) | Final weight(g) | Weight gain (g) |
| Positive Control | 60 | 10.40 ± 2.88ab | 17.68 ± 8.75b | 07.28 ± 5.87 |
| Negative Control | 100 | 11.44 ± 2.92ab | 22.14 ± 9.09ab | 10.70 ± 6.17 |
| Bsp1 | 100 | 7.9 ± 4.07bc | 25.76 ± 8.28ab | 17.86 ± 4.24 |
| Bsp2 | 90 | 3.9 ± 2.2d | 18.38 ± 3.85b | 14.48 ± 1.65 |
| Bsp3 | 100 | 13.82 ± 3.28a | 23.02 ± 4.08ab | 09.20 ± 0.80 |
| Bsp4 | 100 | 9.32 ± 2.8abc | 23.26 ± 7.60ab | 13.94 ± 4.80 |
| Bsp5 | 100 | 12.8 ± 3.0a | 21.02 ± 9.54ab | 08.22 ± 6.56 |
| Bsp9 | 80 | 9.3 ± 4.6abc | 15.18 ± 4.97b | 05.88 ± 0.37 |
| Bsp10 | 100 | 10.46 ± 4.2ab | 31.28 ± 12.50a | 20.82 ± 8.30 |
| Bsp11 | 70 | 4.9 ± 2.8cd | 15.52 ± 3.29b | 10.62 ± 0.49 |
| Bsp12 | 100 | 4.32 ± 1.75cd | 15.18 ± 4.97b | 10.86 ± 3.22 |
| Bsp13 | 100 | 6.76 ± 4.28bc | 20.12 ± 8.64ab | 13.36 ± 4.36 |
Values are mean ± SD, n = 5, values with different alphabet superscript are significant at p ≤ 0.05
Table 2: Survival rate of Clarias anguillaris juveniles and weight gain from In-vivo test during pathogenicity assay for 20 days.
Table 2: Survival rate of Clarias anguillaris juveniles and weight gain from In-vivo test during pathogenicity assay for 20 days.
The result showed that positive control recorded the least survival rate (60%), followed by Bsp11 (70%) and Bsp9 (80%). Bsp2 had 90% survival rate and all the rest recorded 100% including the negative control. There was significant difference (P ≤ 0.05) on the growth rate by Bsp10 with final weight of 31.23 ± 012.50. The body weight increase of the treated fish groups Bsp9, Bsp11 and Bsp12, were lower, but not significantly different (P ≥ 0.05) from the positive control. However, Bsp1, Bsp3, Bsp4, Bsp5, Bsp13 and negative control were not statistically different from each other, but significantly (P ≤ 0.05) different from the positive control. These significant different were evident with high weight gain of these fish groups compared to the positive control. Bsp10 recorded the highest weight gain of 20.82 ± 8.3g in 20 days followed by Bsp1 with 17.86 ± g, Bsp2 (14.48 ± 1.65) and Bsp4 had 13.94g. Bsp9 recorded the least weight gain of 5.88 ± 0.37, even lower than the positive control (7.28 ± 5.87). After subjecting the isolates to molecular identification, table 3 below present 10 successful Bacillus strains identified
| Sample ID | Suggested spp | Accession number | Identity % |
| Bsp1 | Bacillus subtilis | MK085082.1 | 100 |
| Bsp2 | Bacillus subtilis | CP026608.1 | 100 |
| Bsp3 | Bacillus cereus | MN122695.1 | 100 |
| Bsp4 | Bacillus subtilis | MN099359.1 | 100 |
| Bsp5 | Bacillus subtilis | MK085082.1 | 100 |
| Bsp9 | Bacillus cereus | MN122695.1 | 100 |
| Bsp10 | Bacillus subtilis | MN099359.1 | 100 |
| Bsp11 | Bacillus velezensis | CP041145.1 | 100 |
| Bsp12 | Bacillus amyloliquefaciens | MN099360.1 | 100 |
| Bsp13 | Bacillus velezensis | CP041145.1 | 100 |
Table 3: Bacillus species distribution and Percentage similarity of identified strains against reference strains in the Gen Bank.
Discussion
The twelve Bacillus species characterized were Bacillus subtilis (5 strains), B. cereus (2), B. velezensis (3), B. amyloliquefaciens (1) and B. safensis (1). Clostridium sporogenes (1) was also characterized and screened successfully alongside with Bacillus species, probably because, there has been record of its been potential probiotic bacteria (Guo et al., 2020). The isolation, characterization and use of these Bacillus species on fish elsewhere as potential probiotics have been documented by other workers including B. velezensis (Wang et al., 2020), B. subtilis (Hussein et al., 2013; Overview et al., 2014), B. amyloliquifaciens (Afrin et al., 2019) and C.sporogenes (Guo et al., 2020).
The mucosal attachment revealed differences in the adhesion among the Bacillus species. The significant difference (p ≤ 0.05) of better adherence in the mucus of the catfish by B. velezensis (OD620 0.044), B. subtilis (OD6200.034), B. amyloliquefaciens (OD6200.030) corroborates with the works of Ayeni (2011) and Barbosa et al. (2014) who evaluated the functional potential of Weissella and Lactobacillus isolates obtained from Nigeria traditional fermented foods and cow’s intestine in selection process of probiotics. The reduction in population in the course of mucosal attachment corroborate with the studies of Yao et al. (2017) and Yao et al. (2018) who reported the reduction of Lactobacillus salivarius Li01 and Pediococcus pentesaceus Li05 in simulated intestinal fluid. Successful colonization of the GIT is a key factor for probiotic bacteria to be able to exert a sufficient host-interaction to confer health benefits (Yao et al., 2018). The ability of probiotic bacteria to adhere to the intestinal mucosa is considered as one of the main criteria in selection of potential probiotic bacteria since adhesion prolongs their performance in the intestine which in turn allows them to exert beneficial health. Any successfully attached probiotic bacteria to intestinal mucus influences the gastrointestinal microflora of the host which enhances their antagonistic activities against pathogens (Lazado and Caipang, 2014).
Adhesion of probiotic to intestinal mucosa is considered to be associated with several documented health benefits that give the organism an edge to easily colonize the intestine and achieve the desired goal. Chauhan and Singh (2019) iterated the importance of adhesion of probiotic to intestinal mucosa that the survival in bile, pH and pancreatic enzymes are considered prerequisites for probiotic functionality and these benefits constitute the main selection criteria for probiotics. Therefore, in this study, the significant (p ≤ 0.05) difference recorded increased mucosal attachment compared to control group shows a better potential to survive and proliferate in the GIT environment and effectively discharge the desired probiotic effects.
The varied level of adhesion recorded in the study also was in agreement with Hemaiswarya et al. (2013) who enumerated factors that influence the colonization of microorganisms such as host- and microorganism-related factors. The process of attachment of bacteria to intestinal mucosa involves 2 processes including reversible and stable stages (Han et al., 2021). Widanarni et al. (2015) explained that, “the reversible factor involves probiotic bacteria binding to mucosa through nonspecific physical contact, while “the stable stage involved specific interaction between adhesins and complimentary receptors where the probiotic establish a stable binding to the mucus which successfully colonize the GIT. Adhesion and colonization of mucosal surfaces are possible protective mechanism against pathogens through competition for binding site and nutrient or immune modulation. This attachment or colonization is a good attribute to probiotic bacteria, since by attachment to the intestinal mucosa, probiotics can extend their time within the gut and influence the gastrointestinal microflora of their host (Mohammad et al., 2021). Since the bacteria adhesion to tissue surfaces is important during the initial stages of pathogenic infection, competition for adhesion receptors with pathogens might be the first probiotic effect (Hemaiswaryaet al., 2013). When the concentration of probiotic bacteria is high enough, the harmful bacteria would not colonize the adhesion sites and, thus cannot proliferate to establish infection. Therefore, colonization capacity of these probiotic bacteria is very important in development of resistance against colonization by unwanted microorganisms.
The purpose of the gastrointestinal simulation was to know the survival and concentration of potential probiotic bacteria in the stomach, duodenum and intestine in the presence of enzymes, pH changes and the adherence of the isolates to the luminal mucosa. It was also to assesses the survival rate of bacteria before reaching the intestine which is the site of action of orally administered probiotics. The survival in the chemically simulated GIT transit was studied among the Bacillus species that have undergone selection processes. In general, the isolates showed good survival in the GIT transit with acceptable adhesion except for Bsp11 that grow and acidify milk. The sensitivities of Bsp1, Bsp4, Bsp5 and Bsp11 to the simulated gastric juice (GJ) in all the pH with evident drop in population compared to initial population could serve as an early indication that their population may not be enough at the final action point (intestine), so may not encourage good probiotic effects. However, the resistance of Bsp2, Bsp3, Bsp9, Bsp10, Bsp12 and Bsp13 in the GJ in all the pH levels was an indication of reaching the intestine in a good population to discharge good probiotic activities. The bile concentration of the simulated duodenal juice (DJ) did not affect the activities of almost all the Bacillus species since they multiplied more than those in the GJ. In this study, the resistivity of Bsp9, Bsp10, Bsp12 and Bsp13 were significantly (p ≤ 0.05) good which means that no matter the adverse effect of the GJ, their proliferation in the DJ will cover the lost and good population can still reach the intestine.
The significant (P ≤ 0.05) increase in the multiplication of these Bacillus species in almost all stages except Bsp2, Bsp4, Bsp9 and Bsp11 was a good indication of having good population in the intestine and subsequently good probiotic activities. The record of maintaining a constant or increase in population along the GIT transit and significant multiplication in IJ was a sign of being a good probiotic for oral administration. This implies that as good population of probiotic bacteria reach the intestine; good effect will be achieved such as blocking the adhesion of pathogenic bacteria to the epithelium through inhibitory substances, enhancing the intestinal immune response, repair intestinal permeability and regulating intestinal electrolytes absorption (Ayeni et al., 2011). The survival of these isolates along the GIT transit fulfills some criteria for bacteria to be used as probiotic bacteria must survive the intestinal transit (acid and bile tolerant); must adhere to mucosal surface and colonize the intestines, and it must be stable during processing and storage (Singh et al., 2011).
During this study, numbers of probiotic bacteria ranged between 6-9Log CFUg-1 which agreed with the postulation of Maritiel et al. (2021), that probiotics should be present in food in an amount of 8-9 LogCFUg-1 in the daily recommendation before ingestion to ensure that a sufficient therapeutics minimum of 6-7LogCFUG-1 can reach the colon. But the range obtained in the work of Maritiel et al. (2021) was 2-9LogCFUg-1 recording a wider range than 6-9LogCFUg-1 obtained in this study. However, the study of Hill et al. (2014) affirms that for the probiotic supplement to offer such benefit, it must reach the ileum portion of the small intestine in a minimum concentration of 6LogCFUg-1. So, in this study, Bsp3, Bsp5, Bsp9, Bsp10 and Bsp13 showed good initial concentration of above 8LogCFUg-1 and after activation, passed through the simulated GIT and maintained the concentration above 8LogCFUg-1.
The pathogenicity of these successful isolate on catfish was conducted to scrutinize their effect before using them on catfish for probiotics and as growth promoter. The 90-100% survival rate recorded and good growth performance with significant difference suggests that the isolates selected in this study are safe for use in the catfish and for growth promotions. Globally, especially in these recent years, focus has been shifted from the use of synthetic additive in fish diet for disease control and or growth promotion to the use of probiotics and its products for health purposes. Several probiotics have been used in nutritional studies as growth promoter (Moshen et al., 2016), water quality improvement (Padmavathi et al., 2018), for disease control and prevention (Sihang and Sharma, 2012), anti-stress (Cruz et al., 2012) in the field of aquaculture. There has not been any report on isolation and characterization of probiotic bacteria from the skin and gastrointestinal tract of indigenous catfish for use as disease prevention, growth promotion and immune modulation for good yield.
Conclusion
Among the Bacillus species subjected for selection, Bsp9, Bsp10, Bsp12 and Bsp13 were significantly (p ≤ 0.05) resistant through the git which means that no matter the adverse effect of the GJ, their proliferation in the DJ will cover the lost and good population can still reach the intestine and also an indication of reaching the intestine in a good population to discharge good probiotic activities. With the good mucosal attachment power and growth performance these isolates were selected as potential probiotics. These isolates were identified as Bacillus cereus, Bacillus subtilis, Bacillus amyloliquefaciens and Bacillus velezensis.
References
- Ashraf, S., Zaneb, Z., Yousaf, M.S., Ijaz, A., Sohail, M.U., Muti, S., Usman, M.M., Ijaz, S. and Rehman, H. (2013). Effect of dietary supplementation of prebiotics and probiotics on intestinal microarchitecture in broilers reared under cyclic heat stress. Journal of Animal Physiology and Animal Nutrition. 97: 68–73.
- Afrin, S., Nazrul, M. and Bhuiyan, I. (2019). Antagonistic activity of Bacillus amyloliquefaciens subsp . amyloliquefaciens against multidrug resistant Serratia rubidaea. Pp 1-26
- Ayeni, F.A., Sánchez, B., Adeniyi, B.A., Reyes-Gavilán, C. G., Margolles, A. and Ruas-Madiedo, P. (2011). Evaluation of the functional potential of Weissella and Lactobacillus isolates obtained from Nigerian traditional fermented foods and cow's intestine. International Journal of Food Microbiology, 147: 97-104
- Barbosa, J., Borges, S. and Teixeira, P. (2014). Selection of potential probiotic Enterococcus faecium isolated from Portuguese fermented food. International Journal of Food Microbiology 191 (2014): 144–148
- Chauhan, A. and Singh, R. (2019). Probiotics in aquaculture: a promising emerging alternative approach. Symbiosis 77: 99–113.
- Christiana-Teodor, B., Alina, G.P., Camelia, V. (2014). Effect of probiotic Bacillus species in aquaculture- An overview. food Technology 38(2): 9-17
- Cruz, P. M., Iba´nez, A.L., Hermosillo, O. A. M. and Saad, H.C.R. (2012). Review Article Use of Probiotics in Aquaculture. International Scholarly Research Network in Microbiology Volume 2012, Article ID 916845. Pp 1-13
- Cutting, S. M. (2011). Bacillus probiotics. Food Microbiology. 28: 214–220.
- Edward. A.; Ladu, B.M.B and Elihu, A. (2010). Growth, survival and production economics of Clarias gariepinus fingerlings at different stocking densities in concrete tanks. African Journal of General Agriculture 6(2): 1595-6984.
- Ezema C. (2013). Probiotics in animal production: a review. Journal of Veterinary Medicine and Animal Health. 5(11): 308–316.
- Fouad M. F. Elshaghabee, N. R., Rohini, D. G., Chetan S. and Harsh, P. (2017). Bacillus as Potential Probiotics: Status, Concerns, and Future Perspectives. Frontiers in Microbiology, 8:1490
- Gomez-Gill, B., Roque A. Turn bell J.F (2000). The use and selection of probiotic bacteria for use in the culture of larval aquatic organisms. Aquaculture 19: 259-270
- Guo, P., Zhang, K. and Ma, X. 2020). Clostridium species as probiotics: potentials and challenges. Journal of Animal Science and Biotechnology 11, (24):1-10.
- Han, S., Yanmeng L.u ., Jiaojiao X., Yiqiu F., Guiwen Z., Ziyuan W., Jie L., Longxian L., Zongxin L., Björn B., Mingfei Y.., Lanjuan L. (2021). Probiotic Gastrointestinal Transit and Colonization After Oral Administration: A Long Journey. Front in Cellular Infection and Microbiology.
- Hemaiswarya S., Raja R., Ravikumar, R. and Carvalho I. S. (2013). Mechanism of Action of Probiotics. Brazilian Archives of Biology and Technology an international journal, 56 (1):113-119
- Hussein, T., Roohi, A., Munir, S., Ahmed, I., Khan, J., Kim, K. Y. and Anees, M. (2013). Biochemical characterization and identification of bacterial strains isolated from drinking water sources of Kohat, Pakistan, 7(16), 1579–1590.
- Lazado, C.C., Caipang, C.M.A. and Kieon, V. (2012). Enzymes from the gut bacteria of Atlantic cod Gadus morhua and their influence on intestinal enzyme activity. Aquaculture Nutrition 18: 423-431
- Lazado CC, Caipang CMA. (2014). Mucosal immunity and probiotics in fish. Fish Shellfish Immunol. 39:78-89
- Merrifield, D. L., Dimitroglou, A., Foey, A., Davis, J. S., Bakar, T.M.R., Bogwald, J. Castex, M. and Ringo, E. (2010). The current status and future focus of probiotics and prebiotic application for salmonids. Aquaculture, 302:1-18
- Mohammad, A. S., Md.Ariful, I. , Sulav, I. P., Md.Mahbubur, R. , Mohammad, L. R., Fatama, I., Ashikur, R., Dinesh, C. S., Md.Shah, A. And Tofazzal, I. (2021). Gut probiotic bacteria of Barbonymus gonionotus improve growth, hematological parameters and reproductive performances of the host. Scientifc Reports, 11:10692.
- Monica, K. S. and Jayaraj, E. G. (2021). Review on probiotics as a functional feed additive in aquaculture. International Journal of Fisheries and Aquatic Studies, 9(4): 201-207.
- Moshen, S.H., Ahmed, Z. Nobel, F.A.E., Mohammed, E. and Hanan, A.A. (2016). Effects of different growth promoters on performance, feed utilization and body composition of common carp (Cyprinus carprio). Journal of fisheries and aquatic Science, 11: 370-377
- Mulalo, E. N., Yrielle, R., Neil, G. and Rajesh, L.(2013). The Use and Benefits of Bacillus Based Biological Agents in Aquaculture. Sustainable Aquaculture Techniques INTEC. Pp 1-35
- Ngam, P.I.T. and Phu, T.Q (2011). Effects of Bacillus bacteria (B8, B37, B38) on water quality of black tiger shrimp (Panaeus monodon) cultured tank. Proceedings of the 4th aquaculture and fisheries conference. Pp 28-41
- Olaosebikan, B. D. and Raji, A. (2004). The Family Claridae. In : Field guide to Nigerian Freshwater Fisheries, second edition. Federal College of Freshwater Fisheries technology, New-Bussa. Pp 51-55
- Overview, A. N., ?, C. B., Profir, A. G., & Vizireanu, C. (2014). Effects of probiotic, 38(2): 9–17.
- Oyetayo, V.O. and Oyetayo, F.L. (2005). Potential of probiotics as bio-therapeutic agents targeting the innate immune system. African Journal of Biotechnogy 4(2): 125-127
- Quattara, H. G., Reverchon, S., Niamke, S. L., and Nasser, W. (2017). Regulation of the synthesis of pulp degrading enzymes in Bacillus isolated from cocoa fermentation. Food Microbiol. 63, 255–262.
- Rini, M., Mohammad, A.S., Widanarni and Enang, H. (2014). Isolation, Selection and Application of probiotic bacteria for improvement of the growth performance of Humpback grouper (Cromileptes altvelis). International journal of science: Basic and applied Research 16(1): 364-379
- Ripert, G., Racedo, S. M., Elie, A. M., Jacquot, C., Bressollier, P., and Urdaci, M. C. (2016). Secreted compounds of the probiotic Bacillus clausii strain O/C inhibit the cytotoxic effects induced by Clostridium difficile and Bacillus cereus toxins. Antimicrob. Agents Chemother. 60, 3445–3454.
- Sarkar, M. J. A. and Rashid, M. M. (2012). Pathogenicity of the bacterial isolate Aeromonas hydrophila to catfishes, carps and perch. Journal of Bangladesh Agricultural University, 10(1): 157–161
- Schultz, M., Burton, J.P. and Chanyi, R.M. (2017). Use of Bacillus in Human intestinal probiotic applications. In: Floch MH, Rngel Y, Walker WA (ed) The Microbiota in Gastrointestinal Pathophysiology, 1st edn. Elsevier, Amsterdam. 119-123.
- Sihag, C.R. and Sharma, P. (2012). Probiotics: The New Ecofriendly Alternative Measures of Disease Control for Sustainable Aquaculture. Journal of Fisheries and Aquatic Science, 7: 72-103.
- Singh, K., Kallali, B., Kumar, A. and Thaker, V. (2011). Probiotics: A review. Asian Pacific Journal of Tropical Biomedicine, 1(2): 287-290.
- Takano, H. (2016). The regulatory mechanism underlying light-inducible production of carotenoids in non phototrophic bacteria. Biosci. Biotechnol. Biochem. 80, 1264–1273.
- Vesterlund, S., Paltta, J., Karp, M., Ouwehand, A.C.,( 2005). Measurement of bacterial adhesion in-vitro evaluation of different methods. Journal of Microbiological Methods 60, 225–233.
- Vine, N.G., Leukes, W.D., Kaiser, H., Daya, S., Boxter, J., Hecht, T. (2004). Competition for attachment of aquaculture candidate probiotic and pathogenic bacteria on fish intestinal mucus. Journal of Fish Diseases, 27: 319-326
- Wang Y., Jiang Y., Deng Y., Yi C., Wang Y., Ding M., Liu J., Jin X., Shen L., He Y., Wu X., Chen X., Sun C., Zheng M., Zhang R., Ye H., An H. and Wong A. (2020). Probiotic Supplements: Hope or Hype? Frontier in Microbiology, 11(160): 1-9.
- Ronan, O.T., Susanne, L., Sten-Ake, F., Toole, O.R., Lundberg, S., Fredricksson, S., Jansson, A., Nilson, B. and wolf-Watz, H (1999). The chemotactic response of Vibrio anguilarum to fish intestinal mucus is mediated by combination of multiple mucus components. Journal of Bacteriology. Pp 4308-4317
- Sahadeva, R.P.K., Leong, S.F., Chua, K.H., Tan, C.H., Chan,H.Y., Tong, E.V., Wong, S.Y.W. and Chan, H.K. (2011). Survival of commercial probiotic strains to pH and bile. International Food Research Journal, 18: 1515–1522.
- Widanarni , T. N. And Dedi J. (2015). Screening of Probiotic Bacteria Candidates from Gastrointestinal Tract of Pacific White Shrimp Litopenaeus vannamei and their Effects on the Growth Performances. Research Journal of Microbiology, 10: 145-157.
- Yao, M. F., Wu, J., Li, B., Xiao, H., McClements, D. J., and Li, L. J. (2017). Microencapsulation of Lactobacillus salivarious Li01 for enhanced storage viability and targeted delivery to gut microbiota. Food Hydrocolloids 72, 228–236.
- Yao, M., HaoweiYe, B.H., Huang, W., Luo, Q., Xiao, H., McClements, D.J. and Li, L. (2018). Enhanced viability of probiotics (Pediococcus pentosaceus Li05) by encapsulation in microgels doped with inorganic nanoparticles. Food Hydrocolloids. 83: 246-252
Citation: Kolndadacha Dahenji Oscar and Akwuobu Chinedu Adiev. (2025). Selection of Probiotic Bacteria from some Bacillus Species Isolated from the Skin and Gastrointestinal Tract of Catfish (Clarias Anguillaris) in Makurdi Metropolitan, Nigeria. Archives of Veterinary and Animal Sciences 7(1).
Copyright: © 2025 Kolndadacha Dahenji Oscar. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
