Research Article
Volume 6 Issue 1 - 2024
Seroprevalence and Associated Risk Factors of Foot and Mouth Disease in Cattle and Goats of Makawanpur District, Nepal
1Veterinary Hospital and Livestock Service Expert Center, Hetauda, Makawanpur, Nepal
2Agriculture and Forestry University; Faculty of Veterinary Science, Animal Science and Fisheries, Rampur, Chitwan, Nepal
3United Veterinary Center, Hetauda, Makawanpur, Nepal
2Agriculture and Forestry University; Faculty of Veterinary Science, Animal Science and Fisheries, Rampur, Chitwan, Nepal
3United Veterinary Center, Hetauda, Makawanpur, Nepal
*Corresponding Author: Dhurba DC, Veterinary Hospital and Livestock Service Expert Center, Hetauda, Makawanpur, Nepal.
Received: March 25, 2024; Published: April 11, 2024
Abstract
Foot and Mouth Disease virus (FMDv) causes significant economic losses to the Cattle and Goat industry and is considered one of the most economically important viral diseases of cloven-hoofed animals. A cross-sectional seroprevalence study was carried out in cattle and goats of Makawanpur district from three local levels. A total of 650 serum samples (400 from cattle and 250 from goat) were collected from Hetauda Sub-metropolitan city, Manahari Rural Municipality, and Makawanpurgadhi Rural Municipality, and serum samples were tested for antibodies against FMD virus by NSP ELISA. Among 400 cattle serum samples, 83 (20.75%) serum samples were found positive for FMD, and 29 (11.6%) serum samples were found positive to FMD out of 250 goat serum samples. On an age-wise basis, significantly higher seropositivity was found in cattle but not in goats between age groups more than 1 year and less than 1 year. 30 (15.38 %) cattle less than 1 year age was positive and 53 (25.85%) having age group more than 1 year were positive. 10 (9.52%) goat less than 1 year age was positive and 19 (13.1%) having age group more than 1 year were positive to FMD NSP ELISA test. There was a significant difference in the seropositivity of FMD in cohoused and non-cohoused cattle but not in goat. Out of 100 samples were taken from cattle cohoused with goat 28 (28%) samples were positive to FMD (χ2 = 4.262; P<0.05). Similarly in Goat out of 50 samples 10 (20%) samples were positive to FMD (χ2 = 4.301; P>0.05).
Keywords: Foot and Mouth Disease; Makawanpur; Seroprevalence; Serum; NSP ELISA
Introduction
Nepal is an agricultural country. About 65% of the population is engaged in the agriculture and livestock sector contribute about 11% of GDP in Nepal. The total cattle population in Nepal is 7.38 million and the goat population is 12.28 million (MoALD, 2020). Livestock is an integral part of the agricultural economy, and different animal species are being raised, each with their own religious, cultural, nutritional, and agricultural value. People are however unaware of the losses they are bearing due to poor management and hygiene at their livestock farms. Many infectious diseases are prevalent in the livestock population of Nepal as a result of poor hygiene and management.
Foot and mouth disease (FMD) is one of the most significant animal diseases. This disease was first time recorded in 1544 in Europe but the virus was determined by Loffler and Frosch from 1987 to 1900 (Chakraborty, 2000). Among Asian countries, FMD is highly prevalent in India, Burma, and Nepal. This viral disease is caused by Aphthovirus, of the Picornaviridae family of which 7 immunological distinct serotypes O, A, C, Asia 1, SAT 1, SAT 2, and SAT 3 are known (Jha, 2012). In Nepal most O, A, C, and Asia 1 serotype first time identified in 1965, 1967, 1990, and 1984 respectively. Serotype C was observed only during the period from 1990-1996. Since 2012, serotype O is common in Nepal (Khanal et al., 2020). The disease is characterized by fever, loss of appetite, salivation and vesicular eruption of the oral mucosa, skin of inter-digital spaces, and coronary bands of feet and teats (Kibore et al., 2014). The most common sequelae to FMD in cattle are tongue erosion, mastitis, breeding problems, secondary infections, panting, hoof deformities, unthriftiness, abortion, and low milk production (Chakraborty, 2000).
FMD is one of the major transboundary animal diseases (TADs) that causes devastating economic losses and undermines food security. Economic loss estimated due to FMD was NPR 94,806 per household which was mainly concerned with milk production (National FMD control strategy, 2015-2025).
Materials and Methods
Study area
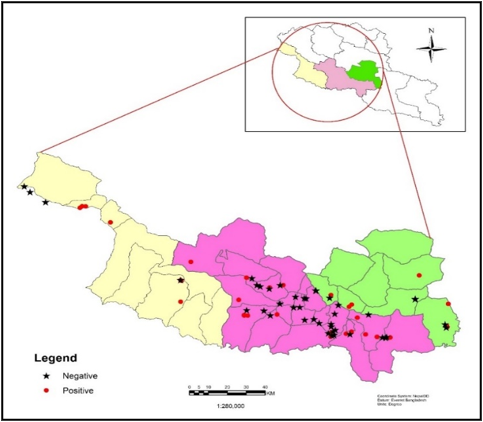
The study was conducted at three different local levels (Hetauda Sub-metropolitan city, Manahari Rural municipality, and Makawanpurgadhi Rural municipality) of Makawanpur district, Nepal. (Figure 1). It is located on latitude 27° 26' N and longitude 85° 17'
The study was conducted at three different local levels (Hetauda Sub-metropolitan city, Manahari Rural municipality, and Makawanpurgadhi Rural municipality) of Makawanpur district, Nepal. (Figure 1). It is located on latitude 27° 26' N and longitude 85° 17'
Study design
The cross-sectional serological survey was conducted from May 2020 to October 2020. in local levels were conveniently selected out of ten local municipalities of Makwanpur district. The study area was selected based on cattle and goat population density and housing system. Competitive ELISA technique (NSP ELISA) was used to detect antibodies to the FMD virus in cattle and goats.
The cross-sectional serological survey was conducted from May 2020 to October 2020. in local levels were conveniently selected out of ten local municipalities of Makwanpur district. The study area was selected based on cattle and goat population density and housing system. Competitive ELISA technique (NSP ELISA) was used to detect antibodies to the FMD virus in cattle and goats.
Sampling method
Openepi, version 3 was used for sample size determination.
n = [DEFF*Np(1-p)]/ [(d2/Z21-α/2*(N-1) +p*(1-p)]
By taking the expected prevalence of 40% for cattle and 15% for goat at a 95% level of confidence and a ±5% desired level of precision in the formula; sample sizes of 366 cattle and 196 goats were determined for the study. But for more accuracy more than the calculated sample size i.e., 650 samples were taken. The sample was collected to compare the seroprevalence based on housing system i.e., cattle and goat cohoused and non-cohoused and that is categorized in two age group, less than one year and more than one year of age.
Openepi, version 3 was used for sample size determination.
n = [DEFF*Np(1-p)]/ [(d2/Z21-α/2*(N-1) +p*(1-p)]
By taking the expected prevalence of 40% for cattle and 15% for goat at a 95% level of confidence and a ±5% desired level of precision in the formula; sample sizes of 366 cattle and 196 goats were determined for the study. But for more accuracy more than the calculated sample size i.e., 650 samples were taken. The sample was collected to compare the seroprevalence based on housing system i.e., cattle and goat cohoused and non-cohoused and that is categorized in two age group, less than one year and more than one year of age.
Sample collection
Blood samples were collected from the jugular vein of individual animals by using a sterile 5 ml syringe. The sample was coded, stored overnight at room temperature for serum separation, and transferred into a sterile serum vial after separation, the sera samples were stored at -20°C until a laboratory test was performed. Then the samples were transported by maintaining a cold chain to the Veterinary Laboratory, Janakpur, Dhanusa, Nepal for further test.
Blood samples were collected from the jugular vein of individual animals by using a sterile 5 ml syringe. The sample was coded, stored overnight at room temperature for serum separation, and transferred into a sterile serum vial after separation, the sera samples were stored at -20°C until a laboratory test was performed. Then the samples were transported by maintaining a cold chain to the Veterinary Laboratory, Janakpur, Dhanusa, Nepal for further test.
All the blood samples were collected from non-vaccinated animals. Out of 650 samples, 150 serum samples (100 samples of cattle and 50 of the goat) were collected from cattle cohoused with the goat. Similarly, 300 serum samples of cattle not cohoused with goat and 250 serum samples of goat not cohoused with cattle were collected.
Laboratory Test
The sample collected was tested by using NSP ELISA as protocol provided by the manufacturer- ID. Vet. This NSP ELISA test detects antibodies produced against non-structural proteins and so that this test can differentiate vaccinated animals from unvaccinated animals.
The sample collected was tested by using NSP ELISA as protocol provided by the manufacturer- ID. Vet. This NSP ELISA test detects antibodies produced against non-structural proteins and so that this test can differentiate vaccinated animals from unvaccinated animals.
Data Management and Analysis
Data analysis was done by using SPSS version 26 software (IBM SPSS Statistics 26). A Chi-square test was used to identify the association between risk factors and prevalence. The confidence level was held at 95% and P < 0.05 was set for statistical significance. The geographic distribution of the disease in the study area was mapped using GIS software Arc GIS 10.2.
Data analysis was done by using SPSS version 26 software (IBM SPSS Statistics 26). A Chi-square test was used to identify the association between risk factors and prevalence. The confidence level was held at 95% and P < 0.05 was set for statistical significance. The geographic distribution of the disease in the study area was mapped using GIS software Arc GIS 10.2.
The test was validated if the mean value of the Negative Control O.D. (ODNC) is greater than 0.7; ODNC> 0.7 and the mean Positive Control O.D. (ODPC) is less than 30 % of the ODNC.; ODPC / ODNC< 0.3. For each sample, the competition percentage (S/N%) was derived from S/N % =
OD sample
ODNC × 100. If the S/N % ≤ 50 % the test was positive and if S/N % > 50 % the test was negative to FMDV.
ODNC × 100. If the S/N % ≤ 50 % the test was positive and if S/N % > 50 % the test was negative to FMDV.
Results
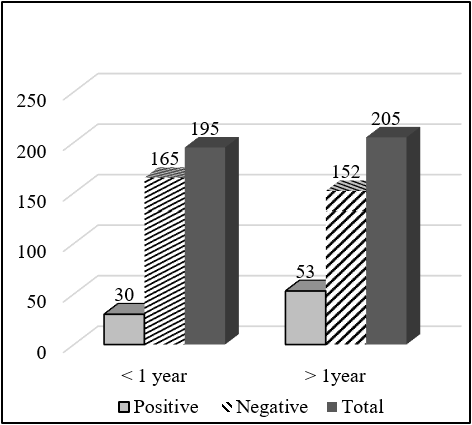
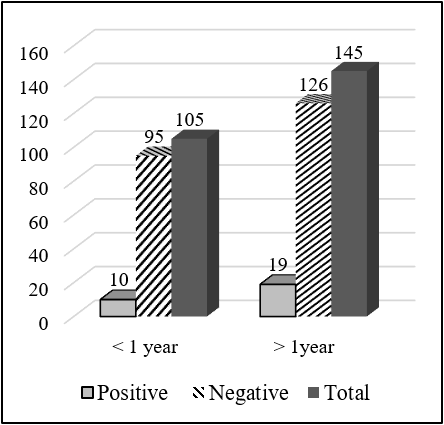
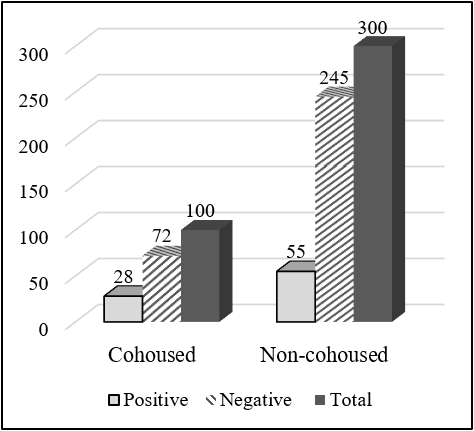
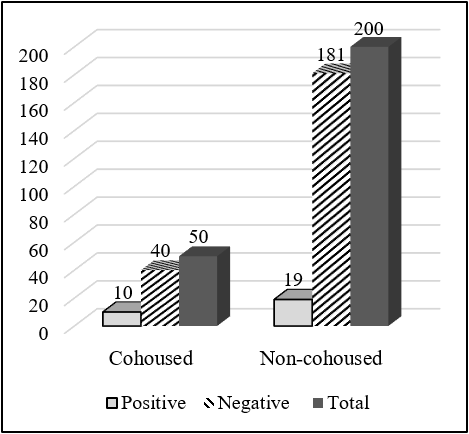
Total 650 serum samples were collected out of which 112 serum samples were found seropositive to FMD from NSP ELISA. Total 83 (20.75%) serum samples were FMDV positive out of 400 cattle serum samples and 29 (11.6%) serum samples positive out of 250 goat serum samples. The total serum samples were grouped into two categories, less than 1 year and more than 1 year. Out of the total sample of cattle having an age group less than 1 year, 30 (15.38%) were positive and 53 (25.85%) having age group more than 1 year were positive to FMD NSP ELISA test. There was a significant difference in the seropositivity between the age group (χ2 = 6.661; P<0.05). Similarly, out of 250 goat serum samples, having an age group less than 1 year, 10 (9.52%) were positive and 19 (13.1%) having an age group more than 1 year were positive. But there was no significant difference in the seropositivity between age groups (χ2 = 0.761; P>0.05). Out of 400 cattle serum samples, 100 samples were taken from cattle cohoused with goat, and the remaining 300 samples were taken from cattle not cohoused with goat and other ruminants. Out of 100 samples, 28 samples were positive to FMD while only 55 samples were positive to FMD out of 300 samples. There was a significant difference in the seropositivity between cohoused and non-cohoused (χ2 =4.262; P<0.05). Out of 250 goat serum samples, 50 samples were taken from cattle cohoused with goat, and the remaining 150 samples were taken from cattle not cohoused with goat and other ruminants. Out of 50 samples, 10 samples were positive to FMD while only 19 samples were positive to FMD out of 150 samples. There was a significant difference in the seropositivity between cohoused and non-cohoused (χ2 = 4.301; P<0.05).
| Variable | Results | Total | OR (Odds Ratio) | ?2 | P-value | |
| Positive (%) | Negative (%) | |||||
| Species | ||||||
| Cattle | 83 (20.75) | 317 (79.25) | 400 | 1.995 | 9.031 | 0.03* |
| Goat | 29 (11.6) | 221 (88.4) | 250 | |||
| Age (Cattle) | ||||||
| < 1 year | 30 (15.38) | 165 (84.62) | 195 | 0.521 | 6.661 | 0.010* |
| >1 year | 53 (25.85) | 152 (74.15) | 205 | |||
| Age (Goat) | ||||||
| < 1 year | 10 (9.52) | 95 (90.48) | 105 | 0.698 | 0.761 | 0.383 |
| >1 year | 19 (13.1) | 126 (86.9) | 145 | |||
| Housing system (Cattle) | ||||||
| Cohoused | 28 (28) | 72 (72) | 100 | 1.732 | 4.262 | 0.039* |
| Non- cohoused | 55 (18.33) | 245 (81.67) | 300 | |||
| Housing system (Goat) | ||||||
| Cohoused | 10 (20) | 40 (80) | 50 | 2.382 | 4.301 | 0.038* |
| Non- cohoused | 19 (9.5) | 181 (90.5) | 200 | |||
Table 1: Sero-positivity of FMD circulating antibodies in cattle and goat to various demographics.
Discussion
Animal-wise distribution of FMD outbreak from 2000-2009 was highest in cattle (42%) followed by buffalo (32%), goats (19%), sheep (4%), and swine (3%) (Jha, 2012). In Sunsari district, Nepal the seroprevalence of FMD was 38% and goat 14 % (Poudel, 2019). In the Amhara region of Ethiopia seroprevalence of FMD in cattle was 14.37% and the goat was 7.10 % (Mesfine et al., 2019). Overall seropositivity in cattle was found to be 55.2% in Northwestern, Nigeria (Babangida et al., 2017). Cattle herd surrounding four Tanzanian national parks showed that the average seropositivity in cattle was 75.2% (Mkama et al., 2014). Out of 3709 cattle serum samples, 52.5% seropositive to FMD was found in Kenya (Kibore et al., 2014). In five different localities of Diyala province, Iraq, 25.33% seroprevalence of antibodies against FMDV was found. In Pakistan, 25.8% of the goat was found positive to FMDV antibodies (Farooq et al., 2017).
Slightly lower seropositivity of FMD circulating antibodies was found in Cattle. Out of 400 sera sample from cattle 83 (20.75%) sera was found positive and 317 (79.25%) sera samples were found negative for FMDV antibody. Similarly, out of 250 goat serum samples, 29 (11.6%) were found positive and 221 (88.4%) were found negative for FMDV antibody by using the NSP ELISA test.
Significantly higher seropositivity of FMD circulating antibodies was found in age group more than 1 year than age group less than 1 year. Out of the total sample of cattle having an age group less than 1 year, 30 (15.38%) were positive and 53 (25.85%) having age group more than 1 year were positive to FMD NSP ELISA test. Similarly, significantly higher seropositivity of FMD circulating antibodies were found in the adult animal (> 1 year) (18.57%) than young animal (<1 year) (7.85%) (Mesfine et al., 2019) (Amhara region, Ethiopia). Northwestern, Nigeria young cattle less than 2 years of age showed a significantly higher level of FMDV antibodies than older cattle more than 2 years of age (Babangida et al., 2017). Similarly, the seropositivity to FMD in a young animal less than 1 year of age was found 30.80%, 1-2 year of age was found 27.50 and more than 2 years of age was found 41.70% this means significantly higher seroprevalence in adult more than 2 years of age compared to that of young animals less than 1 year of age (Kibore et al., 2014).
In five different localities of Diyala province, Iraq high seroprevalence was observed with an age group of more than 4 years old 40.4% compare to age group 2-4 year, 26.3%. Similarly, 7.9% in less than 6 months of age, 10.5% in 7-12 months age group, and 14.9% in 1-2 years age group (Al-Ajeeli et al., 2018). Farooq et. al., (2017) was found that showed that seropositivity to FMDV antibodies is highest in adult goats (28.6%) followed by a young animal (21.4 %) and kids (16. 3%) in Pakistan.
Significantly higher seropositivity to FMD was found in cattle (20.75%) than in goats (11.6%). Similar research in Ethiopia showed that the seroprevalence of FMD in cattle (14.37%) was statistically significantly higher than that of goats (7.10%) (Mesfine et al., 2019).
Cattle cohoused with the goat was significantly higher seropositive to FMDV than not cohoused practice. Out of 100 samples, 28 samples were positive to FMD while only 55 samples were positive to FMD out of 300 samples. Similarly, out of 50 samples, 10 samples were positive to FMD while only 19 samples were positive to FMD out of 200 samples. Similar research in Ethiopia showed that a high correlation in seroprevalence between cattle and goat indicated a possible cross-transmission between these species (Mesfine et al., 2019).
Conclusion
A cross-sectional study was carried out in three local levels (Hetauda sub-metropolitan city, Manahari Rural municipality, and Makwanpurgadhi Rural Municipality) of Makwanpur district to study risk factors associated with FMD in Cattle and Goat. Sample from cohoused cattle and goats showed significantly higher seroprevalence compared to non-cohoused. The study demonstrated that NSP antibodies of FMDV were significantly higher in cattle compared to the goat. Age group more than a year of age showed significantly higher prevalence than age group less than 1 year but in goat no significant association.
In a phase-wise manner for 2015-2025, the National control strategic plan (NCSP) for Foot and Mouth Disease (FMD) in Nepal has been formulated to implement FMD control. In Nepal, the vaccination of cattle and buffalo is common but these results show that circulation of FMDV antibodies in the goat as evident through NSP ELISA could be incriminated for the endemicity of this disease. Inclusion of the goat population in routine vaccination campaigns is strongly recommended. This study suggests to implement FMD control measures in Makawanpur, Nepal, co-housing multiple animal species should be discouraged, and need for further strengthening of FMD surveillance in other susceptible species.
Acknowledgment
The authors wish to acknowledge Veterinary Laboratory, Janakpur, Dhanusa for providing the space and continue support in research activities.
The authors wish to acknowledge Veterinary Laboratory, Janakpur, Dhanusa for providing the space and continue support in research activities.
Conflict of interests
The author should declare no conflict of interests.
The author should declare no conflict of interests.
References
- Al-Ajeeli, K. S., Al-Azawy, A. K., Al-Anbagi, G. K., & Abdul-Rasoul, L. M. S. (2018). Sero prevalence of foot-and-mouth disease in cattle by 3ABC NSPELISA. Indian. J. Natural. Sci, 51(9): 15425-15678.
- Babangida, D., Ibrahim, A. A., Oladayo, F. O., Magaji, A. A., Alkali, B. R., &Jibril, A. H. (2017). Sero Survey of Foot and Mouth Disease Virus Infection in Cattle Crossing Some Major Border States in Northwestern Nigeria. Folia Veterinaria, 61(3): 12-18.
- Chakraborty, A. (2000). A Text Book of Preventive Veterinary Medicine.
- Farooq, U., Irshad, H., Ullah, A., Latif, A., Zahur, A. B., Naeem, K., ...& Rodriguez, L. L. (2017). Sero-prevalence of foot-and-mouth disease in small ruminants of Pakistan. J. Anim. Plant Sci. 27: 1197-1201.
- Jha, V. C. (2012). Situation of foot and mouth disease and its progressive control initiatives in Nepal. Retrieved Aug, 29: 2017.
- Khanal, D. R., Paudel, N., Khanal, S., Prajapati, M., Shrestha, S. P., & Naletoski, M. P. A. I. SEROPREVALENCE OF FOOT AND MOUTH DISEASE VIRUS (A, O and Asia1) ANTIBODIES IN CATTLE, BUFFALO AND PIGS OF NEPAL. In 11th National Workshop on Livestock and Fisheries Research in Nepal (p. 57).
- Kibore, B., Gitao, C. G., Sangula, A., &Kitala, P. (2014). Foot and mouth disease sero-prevalence in cattle in Kenya.
- Mesfine, M., Nigatu, S., Belayneh, N., &Jemberu, W. T. (2019). Sero-Epidemiology of foot and mouth disease in domestic ruminants in Amhara region, Ethiopia. Frontiers in veterinary science, 6: 130.
- Mkama, M., Kasanga, C. J., Sallu, R., Ranga, E., Yongolo, M., Mulumba, M., &Wambura, P. (2014). Serosurveillance of foot-and-mouth disease virus in selected livestock-wildlife interface areas of Tanzania. Onderstepoort Journal of Veterinary Research, 81(2): 1-4.
- MoALD (2020) “Statistical information on Nepalese agriculture 2018/2019”. https://www.moald.gov.np/publication/Agriculture%20Statistics.pdf
- Poudel, A., (2019).Sero-epidemiology of Foot and mouth disease in Sunsari district of Nepal. (Master’s thesis).
Citation: Dhurba DC, Subir Singh, Mohan Prasad Gupta, ICP Tiwari and Ranjana Ranabhat. (2024). Seroprevalence and Associated Risk Factors of Foot and Mouth Disease in Cattle and Goats of Makawanpur District, Nepal. Archives of Veterinary and Animal Sciences 6(1).
Copyright: © 2024 Dhurba DC. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.