Research Article
Volume 6 Issue 1 - 2024
Prophylactic Effects of Three Nutraceuticals Administered Singly or with Intermediate Gumboro Vaccine on Clinicopathological Changes in Pullets Experimentally Infected with Gumboro Virus
1,2Department of Veterinary Medicine, Ahmadu Bello University Zaria, Nigeria
3Department of Veterinary Pathology, Ahmadu Bello University Zaria, Nigeria
3Department of Veterinary Pathology, Ahmadu Bello University Zaria, Nigeria
*Corresponding Author: Joseph, Godwin, Department of Veterinary Medicine, Ahmadu Bello University Zaria, Nigeria.
Received: January 20, 2024; Published: February 08, 2024
Abstract
Infectious bursal disease (IBD) is a viral disease of young chickens that destroys lymphocytes, leading to immunosuppression. This study was conducted to assess the prophylatic effects of three nutraceuticals administered singly or with an intermediate Gumboro vaccine on clinicopathological changes in pullets experimentally infected with a Gumboro virus. A total of 240 ISA Brown pullets were divided into 12 groups, A, B, C1, C2, C3, D1, D2, D3, E1, E2, E3 and F, each comprising of 20 birds at two days of age. Group A served as negative control (no nutraceuticals, no vaccine and no vvIBDV). Group B served as positive control (no nutraceuticals, no vaccine, vvIBDV). Group C received nutraceuticals, vaccine and vvIBDV; the group was subdivided into C1, C2 and C3 and each received King-herb oral solution (KH), Gumbo ND (GND) and Grand HumiVet (GHV), respectively. Group D received nutraceuticals, no vaccine and inoculated with vvIBDV; the group was subdivided into groups D1, D2 and D3 with each receiving KH, GND, and GHV, respectively. Group E received nutraceuticals, was not vaccinated and no vvIBDV; the group was subdivided into E1, E2 and E3 which received KH, GND and GHV, respectively. Group F was vaccinated and inoculated with vvIBDV. Nutraceuticals were administered for 19 days from 5 days of age and vaccinated with intermediate Gumboro vaccine at 11 and 22 days of age. The pullets were inoculated with vvIBDV orally at 29 days of age. Cloacal temperatures (CT) of 10 birds per group were monitored from 3 to 7 days post inoculation (dpi). The birds were observed for clinical signs from 3 to 7 dpi and severity of signs presented in percentages. Two live birds were euthanized, weighed and necropsied at 21, 28, 35 and 42 days of age and the bursae of Fabricius (BF) were removed, weighed and fixed in 10% neutral buffered formalin and processed for histopathology. The vvIBDV induced pyrexia (oC) in positive control (B) (42.30 ± 0.19), which was significantly higher (P ≤ 0.05) compared to groups C2 (41.12 ± 0.08) and C3 (40.98 ± 0.09) at 3 dpi. Clinical signs (%) were not observed in groups C1, C2 and C3 but observed in B (28.67) 3 dpi. At 4 dpi morbidity and mortality rates (%) were not observed in groups C1, C2 and C3 while the rates were 25.00/5.86 in B. At 3 dpi, the live body weights (g) of pullets in group B (253.60 ± 12.25) was significantly lower (P ≤ 0.05) compared to groups C1 (367.30 ± 12.28) or C2 (327.00 ± 5.18). Gross lesions (%) in group B was 38.08 while in C1 it was 2.50, C2 5.83 and C3 4.58 at 3 dpi. Histopathological mean score of BF of C1 (3.45 ± 0.05) and C3 (3.63 ± 0.13) were significantly lower (P ≤ 0.05) compared to B (4.50 ± 0.17). In conclusion, King herb oral solution, Gumbo ND, Grand HumiVet and intermediate Gumboro vaccine reduced the negative effects of vvIBDV infection on cloacal temperature, live body weight, and reduced the severity of clinical signs, morbidity rate, mortality rates and gross and microscopic lesions on the bursa of Fabricius. Farmers could administer nutraceuticals and vaccinate pullets against IBD in to prevent disease caused by vvIBDV. Also they should administered nutraceuticals only to potentiate the efficacy of live IBDV vaccine and not depend on them only for the prevention of vvIBD infection.
Keywords: Clinicopathological; Gumboro vaccine; Nutraceuticals; vvIBDV
Introduction
Gumboro disease also known as infectious bursal disease (IBD) is one of the most devastating viral diseases that destroys lymphocytes, leading to severe immunosuppression (Alkie and Rautenschlein, 2016). Chickens are the primary host for infectious bursal disease virus (IBDV), but antibodies and the virus have also been detected in guinea fowls, ducks, quails, pheasants and ostriches with no signs of disease (Lukert and Saif, 2003). In spite of the extensive use of vaccines, farmers still have to contend with IBD (Besseboua et al., 2015). Many manufacturing companies have their own vaccine specifications (Besseboua et al., 2015). The control of IBD depends on appropriate immunization schedules and maintenance of good hygienic conditions on the farm (Farooq et al., 2003). Vaccinating breeding hens with live attenuated or inactivated virus vaccine control the disease. Induced antibodies are transferred to the young chicks via the egg yolks which protect the newly hatched chicks for the critical first few weeks of life (Wyeth and Cullen, 1976, Orakpoghenor et al., 2021). IBD is characterized by high mortality in chicks that are between 3 and 6 weeks old. Studies have shown that, IBD has acquired an endemic status in poultry in Nigerian farms (Nawathe et al., 1978; Durojaiye et al., 1984; Abdu, 1988). To date, there is no known chemotherapeutic agent that is effective in the control of IBD. The severe clinical signs and high mortality rate that results from vvIBDV infection in susceptible chickens, occur due to biochemical changes that occur in relation to the pathological effect of the virus in several organs such as the BF, spleen, thymus, liver and kidneys (Ley et al., 1983; Nunoya et al., 1992; Lukert and Saif, 1997). King-Herb oral solution® was found to be effective in broilers chickens as it ameliorated clinicopathological changes (Joseph, 2019). Effective nutraceuticals in the market with vaccination could reduce the economic losses due to IBD in the poultry industry (Musa et al., 2012). This study was to determine the prophylactic effects of three nutraceuticals administered singly or with intermediate Gumboro vaccine on clinicopathological changes in pullets experimentally infected with Gumboro virus.
Materials and Methods
The Study location
The experiment was conducted at the Poultry Research Pen, of the Department of veterinary Public Health and Preventive Medicine, Ahmadu Bello University in Zaria. The study was carried out during the dry (November-April) season which is characterized by low 13.8oC temperature (Ileojo, 2004). The area has a tropical Savannah climate with annual total rainfall of about 1,099 mm (Ileojo, 2004). Ethical clearance to conduct the study was sought and approved by the Ahmadu Bello University Committee on Animal Use Care (ABUCAUC/2022/008).
The experiment was conducted at the Poultry Research Pen, of the Department of veterinary Public Health and Preventive Medicine, Ahmadu Bello University in Zaria. The study was carried out during the dry (November-April) season which is characterized by low 13.8oC temperature (Ileojo, 2004). The area has a tropical Savannah climate with annual total rainfall of about 1,099 mm (Ileojo, 2004). Ethical clearance to conduct the study was sought and approved by the Ahmadu Bello University Committee on Animal Use Care (ABUCAUC/2022/008).
Experimental chicks and care
A total of 240 pullets were randomly selected and divided at two days of age, into 12 groups A, B, C1, C2, C3, D1, D2, D3, E1, E2, E3 and F comprising of 20 birds each. Group A was negative control (no nutraceuticals, no vaccine, no vvIBDV). Group B was positive control (no nutraceuticals, no vaccine, vvIBDV). Group C had nutraceuticals, vaccine and vvIBDV. The group was sub divided into C1, C2 and C3 for KH, GND, and GHV, respectively. Group D had nutraceuticals, not vaccine and vvIBDV. The group was subdivided into groups D1, D2 and D3 for KH, GND, and GHV, respectively. Group E had nutraceuticals, no vaccine, and no vvIBDV. The group was subdivided into E1, E2 and E3 for KH, GND and GHV, respectively. Group F was vaccinated and inoculated with vvIBDV. The chicks were administered nutraceuticals for 21 days from 5 days of age and vaccinated with intermediate Gumboro vaccine at 11 and 22 days of age. Live body weight of pullets in grams was weight using digital weighing balance. The pullets were inoculated orally at 29 days of age with a vvIBDV. Cloacal temperatures of 10 birds per group were monitored at 3 to 7 dpi. The chicks were observed for clinical signs from 3 to 7 dpi. Dead birds were weighed and necropsied. Two live birds from each group were euthanized by jugular exsanguination, weighed and necropsied at 21, 28, 35 and 42 days of age and the BF, collected weighed and fixed in buffered 10% neutral formalin and processed for histopathology.
A total of 240 pullets were randomly selected and divided at two days of age, into 12 groups A, B, C1, C2, C3, D1, D2, D3, E1, E2, E3 and F comprising of 20 birds each. Group A was negative control (no nutraceuticals, no vaccine, no vvIBDV). Group B was positive control (no nutraceuticals, no vaccine, vvIBDV). Group C had nutraceuticals, vaccine and vvIBDV. The group was sub divided into C1, C2 and C3 for KH, GND, and GHV, respectively. Group D had nutraceuticals, not vaccine and vvIBDV. The group was subdivided into groups D1, D2 and D3 for KH, GND, and GHV, respectively. Group E had nutraceuticals, no vaccine, and no vvIBDV. The group was subdivided into E1, E2 and E3 for KH, GND and GHV, respectively. Group F was vaccinated and inoculated with vvIBDV. The chicks were administered nutraceuticals for 21 days from 5 days of age and vaccinated with intermediate Gumboro vaccine at 11 and 22 days of age. Live body weight of pullets in grams was weight using digital weighing balance. The pullets were inoculated orally at 29 days of age with a vvIBDV. Cloacal temperatures of 10 birds per group were monitored at 3 to 7 dpi. The chicks were observed for clinical signs from 3 to 7 dpi. Dead birds were weighed and necropsied. Two live birds from each group were euthanized by jugular exsanguination, weighed and necropsied at 21, 28, 35 and 42 days of age and the BF, collected weighed and fixed in buffered 10% neutral formalin and processed for histopathology.
Experimental chickens
The number of chickens to be used was determined using G-power (Hinks et al., 2022). Two hundred and forty day-old ISA Brown pullets were purchased from a hatchery in Ibadan, Nigeria. The birds were randomly divided into twelve groups of twenty chicks each and housed on deep litter with floor space of 0.14 m2 per bird.
The number of chickens to be used was determined using G-power (Hinks et al., 2022). Two hundred and forty day-old ISA Brown pullets were purchased from a hatchery in Ibadan, Nigeria. The birds were randomly divided into twelve groups of twenty chicks each and housed on deep litter with floor space of 0.14 m2 per bird.
Housing
The Poultry Research Pen was divided into twelve separate compartments, each housing 20 birds, made of wooden frame-work supported with wire-mesh. Formalin 10% was used to sprayed the pen and the poultry premises (Abdu and Musa, 2014). Strict bio-security sanitation, traffic control and isolation was adhered to throughout the period of research.
The Poultry Research Pen was divided into twelve separate compartments, each housing 20 birds, made of wooden frame-work supported with wire-mesh. Formalin 10% was used to sprayed the pen and the poultry premises (Abdu and Musa, 2014). Strict bio-security sanitation, traffic control and isolation was adhered to throughout the period of research.
Feeding and watering
Feed was purchased from an accredited large distributor of a reputable commercial feed mill. It was provided ad libitum using galvanized feeders. Fresh clean water from a borehole was provided ad libitum in plastic drinkers (4 L) for 20 pullets.
Feed was purchased from an accredited large distributor of a reputable commercial feed mill. It was provided ad libitum using galvanized feeders. Fresh clean water from a borehole was provided ad libitum in plastic drinkers (4 L) for 20 pullets.
Brooding and lighting
A charcoal brooding cupola was used per compartment, for brooding the chicks for a period of one week to provide the 28- 34°C brooding temperature for the chicks. One two hundred watt bulbs was used as source of illumination per compartment.
Live body weight of pullets
Live body weight of pullets in grams was weight using digital weighing balance.
A charcoal brooding cupola was used per compartment, for brooding the chicks for a period of one week to provide the 28- 34°C brooding temperature for the chicks. One two hundred watt bulbs was used as source of illumination per compartment.
Live body weight of pullets
Live body weight of pullets in grams was weight using digital weighing balance.
Challenge virus
A very virulent IBDV was obtained from the Department of Veterinary Medicine, ABU Zaria. The vvIBDV came from previously vaccinated commercial layers that died of a natural outbreak of IBD. Seventy-five per cent (75%) of commercial cockerels inoculated with the vvIBDV at 30 days of age with 50 µL of bursal suspension (v/w) in phosphate buffered saline (PBS) (pH 7.4) died. One millilitre of bursal suspension (v/w) in PBS (pH 7.4) contained 109.76 CID50 of IBDV (Abdu et al., 2007).
A very virulent IBDV was obtained from the Department of Veterinary Medicine, ABU Zaria. The vvIBDV came from previously vaccinated commercial layers that died of a natural outbreak of IBD. Seventy-five per cent (75%) of commercial cockerels inoculated with the vvIBDV at 30 days of age with 50 µL of bursal suspension (v/w) in phosphate buffered saline (PBS) (pH 7.4) died. One millilitre of bursal suspension (v/w) in PBS (pH 7.4) contained 109.76 CID50 of IBDV (Abdu et al., 2007).
Nutraceuticals
King-Herbs oral solution® (KH) is a herbal extracts recommended for the prevention and treatment of viral diseases such as IBD, ND. It is to, induce bodies to form interferon, enhance body's immunity, promote the formation of antibody and improve the activity of macrophages. It also to enhance the antiviral and antibacterial effects to infectious diseases, adjust intestinal function, improve the growth of probiotics, protect intestinal mucosa, increase feed intake and promote growth. The nutraceuticals was administered at 1 ml to 1 litre of drinking water for 19 days starting at 5 days of age. Gumbo ND® (GND) is a contain Sal-ammoniac, Acidiumboricum, Sodium 2 hydroxy benzoate, D-glucitol, L-Ascorbic acid, L-2-amino-4-(methylthio) butyric acid, bromhexine HCL, and Astragalus polysaccharide. It is indicated for prevention and treatment of viral diseases such as IBD and ND. It was administered at 1 ml per litre of drinking water for 19 days starting at 5 days of age. Grand HumiVet® (GHV) is a natural organic dietary supplement containing chelated essential minerals, trace elements and amino acids to improve and maintain health and performance of poultry and livestock. One 100 gram of the natural growth stimulant, antibacterial, anti-mold, antiviral and anti-inflammatory health routine supplement has sodium humate salt (14.0%), humic acid (76.0%), and organic matter (92.0%). It is recommended to enhance immunity during vaccination, minimize the effect of stress, disease prevention and control in the presence of endemic viral infection and normal prevention and routine health measure. The GHV was administered at 1 gram to 1 L of drinking water for 19 days starting at 5 days of age.
King-Herbs oral solution® (KH) is a herbal extracts recommended for the prevention and treatment of viral diseases such as IBD, ND. It is to, induce bodies to form interferon, enhance body's immunity, promote the formation of antibody and improve the activity of macrophages. It also to enhance the antiviral and antibacterial effects to infectious diseases, adjust intestinal function, improve the growth of probiotics, protect intestinal mucosa, increase feed intake and promote growth. The nutraceuticals was administered at 1 ml to 1 litre of drinking water for 19 days starting at 5 days of age. Gumbo ND® (GND) is a contain Sal-ammoniac, Acidiumboricum, Sodium 2 hydroxy benzoate, D-glucitol, L-Ascorbic acid, L-2-amino-4-(methylthio) butyric acid, bromhexine HCL, and Astragalus polysaccharide. It is indicated for prevention and treatment of viral diseases such as IBD and ND. It was administered at 1 ml per litre of drinking water for 19 days starting at 5 days of age. Grand HumiVet® (GHV) is a natural organic dietary supplement containing chelated essential minerals, trace elements and amino acids to improve and maintain health and performance of poultry and livestock. One 100 gram of the natural growth stimulant, antibacterial, anti-mold, antiviral and anti-inflammatory health routine supplement has sodium humate salt (14.0%), humic acid (76.0%), and organic matter (92.0%). It is recommended to enhance immunity during vaccination, minimize the effect of stress, disease prevention and control in the presence of endemic viral infection and normal prevention and routine health measure. The GHV was administered at 1 gram to 1 L of drinking water for 19 days starting at 5 days of age.
Vaccine and vaccination
Gumboro disease vaccine Izovac (live intermediate Gumboro disease virus strain Winterfield 2512,103 EID50 was purchased from a veterinary distributor. It was reconstituted at 500 doses to 5 litres of drinking water and administered at 11 and 22 of age.
Gumboro disease vaccine Izovac (live intermediate Gumboro disease virus strain Winterfield 2512,103 EID50 was purchased from a veterinary distributor. It was reconstituted at 500 doses to 5 litres of drinking water and administered at 11 and 22 of age.
Clinical observation
The number of birds that showed clinical signs such as depression, diarrhoea, prostration, recumbency or inappetance per day in each group was recorded in percentages (%) (Babiker and Tawfeeg, 2008).
The number of birds that showed clinical signs such as depression, diarrhoea, prostration, recumbency or inappetance per day in each group was recorded in percentages (%) (Babiker and Tawfeeg, 2008).
Determination of morbidity and mortality rates
Morbidity and mortality rates was calculated as desbribed by Babiker et al., 2008 using the formulae:
Morbidity and mortality rates was calculated as desbribed by Babiker et al., 2008 using the formulae:
Gross pathology
Following inoculation of pullets with vvIBDV birds that died from 2 to 7 days pi were examined for the presence of gross lesions. Also, two live pullets from each group, were humanely euthanized by jugular exsanguination on days 0, 3 and 7 dpi and examined for gross lesions (OIE, 2004). The organs that were examined include; breast, leg, and thigh muscles BF, spleen, thymus and caecal tonsils, lungs and trachea, pancreas, liver and proventriculus. Photographs of the lesions observed in the bursa of Fabricius were taken using digital camera (Infinix x652B China). The lesions on each organ were scored and graded based on the criteria described by Ward and Thoolen (2011) with modification; congestion (1), oedema (2), transudate (3), enlargement (4), turgid (5), haemorrhage (6), pale (7) and atrophy (8). The gross lesion scores obtained for the different organs of each bird were summed up and divided by the number of lesions to get the score for that group.
Following inoculation of pullets with vvIBDV birds that died from 2 to 7 days pi were examined for the presence of gross lesions. Also, two live pullets from each group, were humanely euthanized by jugular exsanguination on days 0, 3 and 7 dpi and examined for gross lesions (OIE, 2004). The organs that were examined include; breast, leg, and thigh muscles BF, spleen, thymus and caecal tonsils, lungs and trachea, pancreas, liver and proventriculus. Photographs of the lesions observed in the bursa of Fabricius were taken using digital camera (Infinix x652B China). The lesions on each organ were scored and graded based on the criteria described by Ward and Thoolen (2011) with modification; congestion (1), oedema (2), transudate (3), enlargement (4), turgid (5), haemorrhage (6), pale (7) and atrophy (8). The gross lesion scores obtained for the different organs of each bird were summed up and divided by the number of lesions to get the score for that group.
Histopathology
For histopathological examination of lesions, bursa of Fabricius was trimmed to a thickness of 5 mm to 1 cm in size and fixed in 10% neutral buffered formalin. The tissue samples were subjected to different steps of fixation and dehydration in a series of alcohol concentrations (70%, 80%, 90%, 100%) and then cleared by xylene I and II. Thereafter, the tissues were impregnated in paraffin wax at temperature of 60-62 oC and embedded in molten wax. The embedded tissues were sectioned by rotary microtome with thickness of 5 micrometres (Khenenuo et al., 2017). The sectioned ribbon was added to floating bath and picked up by slide and labeled. The labeled slide was stained with Meyer’s Haematoxylin and Eosin dye and after being stained, it was mounted with Canada 18 balsam. The microscopic changes were examined at different magnifications and the histopathological changes were recorded. Tissue was histopathologically evaluated on the basis of the extent of necrosis and degeneration of follicular lymphocytes, the presence of follicles and oedema, hyperemia, and heterophilic infiltration (Bennoune, 2011). The score criteria for BF were; no lesion (0), lymphocyte depletion/ vacuolation (1), infiltrations (2), oedema (3), haemorrhage (4), cyst formation (5) and thickened interfollicular space (6). The histopathology score for a specific nutraceutical in the same group was obtained by adding the score for the different birds divided by the number of the birds examined in that group.
For histopathological examination of lesions, bursa of Fabricius was trimmed to a thickness of 5 mm to 1 cm in size and fixed in 10% neutral buffered formalin. The tissue samples were subjected to different steps of fixation and dehydration in a series of alcohol concentrations (70%, 80%, 90%, 100%) and then cleared by xylene I and II. Thereafter, the tissues were impregnated in paraffin wax at temperature of 60-62 oC and embedded in molten wax. The embedded tissues were sectioned by rotary microtome with thickness of 5 micrometres (Khenenuo et al., 2017). The sectioned ribbon was added to floating bath and picked up by slide and labeled. The labeled slide was stained with Meyer’s Haematoxylin and Eosin dye and after being stained, it was mounted with Canada 18 balsam. The microscopic changes were examined at different magnifications and the histopathological changes were recorded. Tissue was histopathologically evaluated on the basis of the extent of necrosis and degeneration of follicular lymphocytes, the presence of follicles and oedema, hyperemia, and heterophilic infiltration (Bennoune, 2011). The score criteria for BF were; no lesion (0), lymphocyte depletion/ vacuolation (1), infiltrations (2), oedema (3), haemorrhage (4), cyst formation (5) and thickened interfollicular space (6). The histopathology score for a specific nutraceutical in the same group was obtained by adding the score for the different birds divided by the number of the birds examined in that group.
Data Analyses
Data was entered into and stored in Microsoft Excel 2007. The data were exported to Graph pad Prism version 5.03 data base where descriptive statistics was carried out. The data were summarized into percentages, graphs and bar charts. The data were subjected to one-way ANOVA to compare data between groups while variations among groups was determined using Tukey’s comparative test and results express as mean (± SEM). The difference between groups was considered to be significant if P values are less than 0.05.
Data was entered into and stored in Microsoft Excel 2007. The data were exported to Graph pad Prism version 5.03 data base where descriptive statistics was carried out. The data were summarized into percentages, graphs and bar charts. The data were subjected to one-way ANOVA to compare data between groups while variations among groups was determined using Tukey’s comparative test and results express as mean (± SEM). The difference between groups was considered to be significant if P values are less than 0.05.
Results
Clinical signs observed
Clinical signs (%) were not observed in pullets in groups C1, C2 and C3 administered KH, GND, and GHV vaccinated with Gumboro vaccine and inoculated with vvIBDV while 28.67 in group B that did not receive nutraceuticals but inoculated with vvIBDV showed clinical signs at 3 dpi.
Clinical signs (%) were not observed in pullets in groups C1, C2 and C3 administered KH, GND, and GHV vaccinated with Gumboro vaccine and inoculated with vvIBDV while 28.67 in group B that did not receive nutraceuticals but inoculated with vvIBDV showed clinical signs at 3 dpi.
Changes in cloacal temperature
At 3 dpi the cloacal temperature (°C) of pullets in group C3 administered GHV (40.98 ± 0.09) and in C2 administered GND (41.12 ± 0.08), vaccinated with Gumboro vaccine and inoculated with vvIBDV were significantly lower (P ≤ 0.05) than in group B that did not received nutraceuticals but were inoculated (42.30 ± 0.19).
At 3 dpi the cloacal temperature (°C) of pullets in group C3 administered GHV (40.98 ± 0.09) and in C2 administered GND (41.12 ± 0.08), vaccinated with Gumboro vaccine and inoculated with vvIBDV were significantly lower (P ≤ 0.05) than in group B that did not received nutraceuticals but were inoculated (42.30 ± 0.19).
Morbidity and mortality rates
At 4 dpi morbidity and mortality rates (%) were not recorded in pullets in groups, C1, C2 and C3, administered KH, GND and GHV, vaccinated with Gumboro vaccine and inoculated with vvIBDV while the rates were 25.00/5.88 in group B that did not receive nutraceuticals but were inoculated.
At 4 dpi morbidity and mortality rates (%) were not recorded in pullets in groups, C1, C2 and C3, administered KH, GND and GHV, vaccinated with Gumboro vaccine and inoculated with vvIBDV while the rates were 25.00/5.88 in group B that did not receive nutraceuticals but were inoculated.
Changes in live body weight
At 3 dpi the live body weights (g) of pullets in groups, C1, C2 and C3 administered KH (367.30 ± 12.28), GND (346.10 ± 9.48) and GHV (327.00 ± 5.18), vaccinated with Gumboro vaccine and inoculated with vvIBDV were significantly higher (P ≤ 0.05) compared to group B that did not receive nutraceuticals but inoculated (253.60 ± 12.25).
At 3 dpi the live body weights (g) of pullets in groups, C1, C2 and C3 administered KH (367.30 ± 12.28), GND (346.10 ± 9.48) and GHV (327.00 ± 5.18), vaccinated with Gumboro vaccine and inoculated with vvIBDV were significantly higher (P ≤ 0.05) compared to group B that did not receive nutraceuticals but inoculated (253.60 ± 12.25).
Gross lesions observed
At 3 dpi the gross lesions (%) of pullets in groups C1, C2 and C3 administered KH (2.50), GND (5.83) and GHV (4.58), vaccinated with Gumboro vaccine and inoculated with vvIBDV were lower than the value of 38.08 in group B that did not receive nutraceuticals but inoculated with vvIBDV.
At 3 dpi the gross lesions (%) of pullets in groups C1, C2 and C3 administered KH (2.50), GND (5.83) and GHV (4.58), vaccinated with Gumboro vaccine and inoculated with vvIBDV were lower than the value of 38.08 in group B that did not receive nutraceuticals but inoculated with vvIBDV.
Histopathological changes in the of bursa of Fabricius
At 3 dpi the mean histopathological scores of pullets in groups C1 and C3 administered KH (3.45 ± 0.05) and GHV (3.63 ± 0.13), vaccinated with Gumboro vaccine and inoculated with vvIBDV were significantly lower (P ≤ 0.05) compared to group B (4.50 ± 0. 17) that did not receive nutraceuticals but were inoculated.
At 3 dpi the mean histopathological scores of pullets in groups C1 and C3 administered KH (3.45 ± 0.05) and GHV (3.63 ± 0.13), vaccinated with Gumboro vaccine and inoculated with vvIBDV were significantly lower (P ≤ 0.05) compared to group B (4.50 ± 0. 17) that did not receive nutraceuticals but were inoculated.
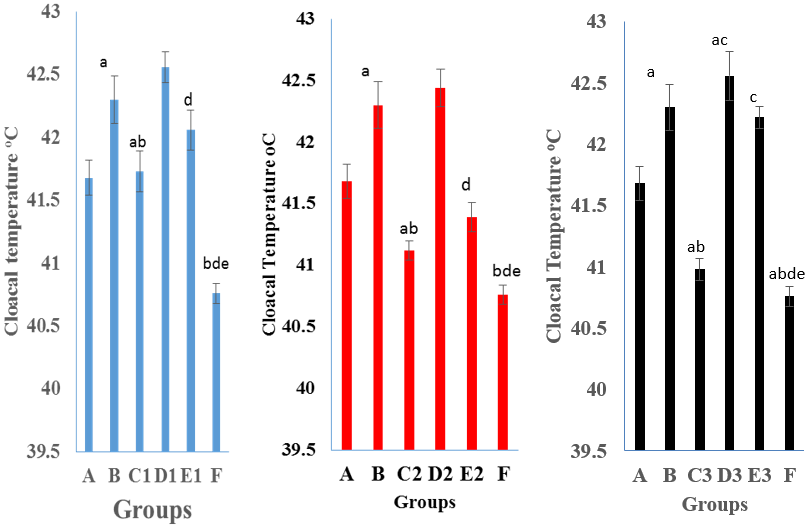
Figure 1: The mean cloacal temperature (°C) of pullets administered nutraceuticals singly or with intermediate Gumboro vaccine experimentally infected with Gumboro virus at 3 dpi.
Key: Key: A Negative control; B Positive control; C1, D1 and E1, were administered King herb oral solution; C2, D2 and E2 – were administered Gumbo ND; C3, D3 and E3 – were administered Grand humiVet; F – Vaccinated and inoculated with vvIBDV. Means (± SEM) with different alphabet in the differ significantly at P < 0.05
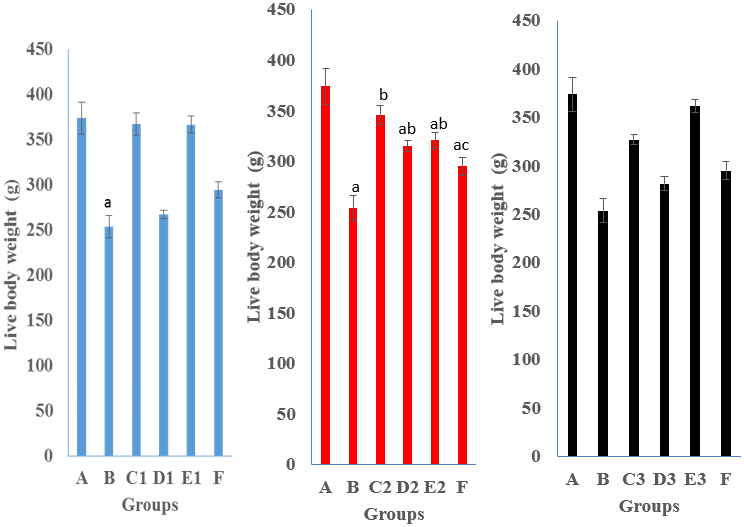
Figure 2: Live body weight (g) of pullets administered nutraceuticals singly or with intermediate Gumboro vaccine and experimentally infected with Gumboro virus at 3 dpi.
Key: A Negative control; B Positive control; C1, D1 and E1 were administered King herb oral solution; C2, D2 and E2 were administered Gumbo ND; C3, D3 and E3 were administered Grand humiVet; F Vaccinated and inoculated with vvIBDV. Means (± SEM) with different alphabet differ significantly at P < 0.05
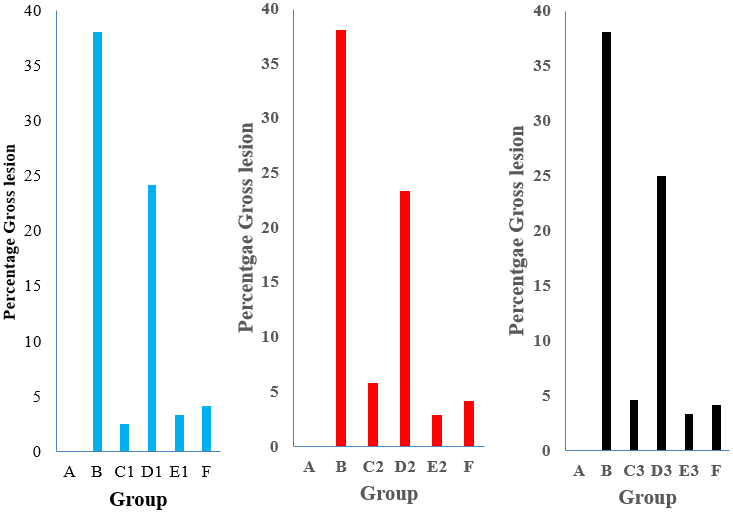
Figure 3: The gross lesions (%) of pullets administered nutraceuticals singly or with intermediate Gumboro vaccine experimentally infected with Gumboro virus at 3 dpi.
Key: A – Negative control; B – Positive control; C1, D1 and E1 – were administered King herb oral solution; C2, D2 and E2 – were administered Gumbo ND; C3, D3 and E3 – were administered Grand humi Vet; F – Vaccinated and inoculated with vvIBDV
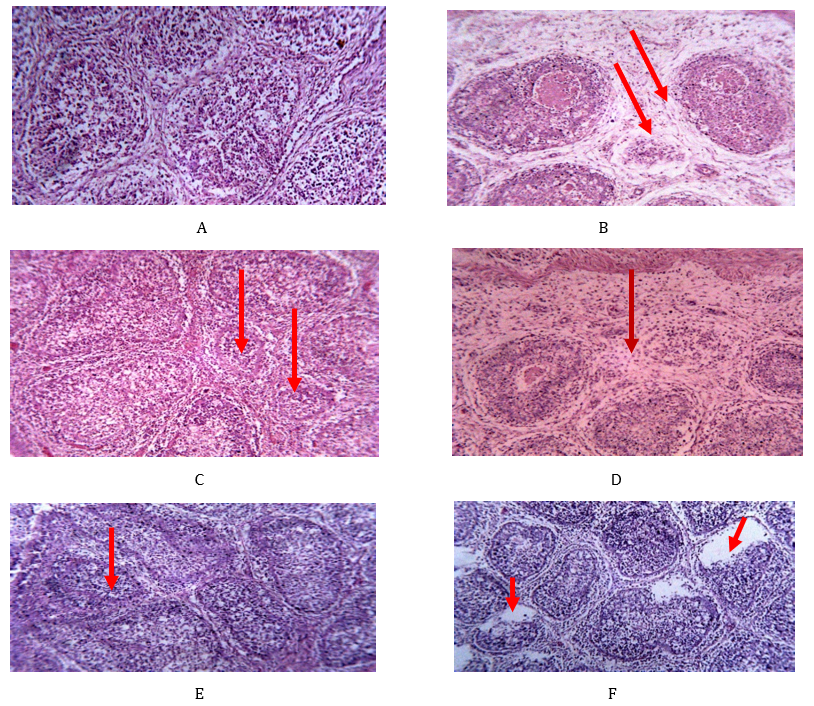
Plate IV: Photomicrographs of sections of bursae of Fabricius of pullets. (A) (negative control) showing normal bursal architecture. (B) thickened interfollicular space filled with edema fluid and mononuclear cells (arrows), and necrotic debris in the follicles. (C1) atrophy of the follicles and infiltration of mononuclear cells (arrows). (D1) necrosis of the follicles, widened interfollicular space filled with oedema fluid and mononuclear cells (arrows). (E1) depletion of follicles and infiltration of mononuclear cells. (F) necrosis and vaculation of follicles (arrows) and infiltration with mononuclear cells. H & E stain x 100 (at 3 days’ post-inoculation).
Discussion
At 3 dpi, the lowest percentage clinical signs were observed in the group administered Gumbo ND and not vaccinated D2 (45.53%) possibly because of the vitamin C present in Gumbo ND which prevent lipid peroxidation observed in vvIBDV (Ertekin et al., 2016).The clinical signs observed in the study which included; ruffled feathers, depression, diarrhoea and somnolence are the common signs seen reported in IBD (Igwe, 2017). In group F clinical signs were observed even though the birds in the group were vaccinated against IBDV. This might be because the antibody titre needed to neutralize all the vvIBDV was low in some birds.
In this study, there was pyrexia in vvIBDV inoculated pullets in the early stages (3 days) of the infection as observed in groups B, D1, D2 and D3. The elevated cloacal temperature occurred likely because there was viraemia in the pullets following IBDV infection (Chevile, 1967; Cho and Edgar, 1972). The pullets body has pyrogens receptors and when the body detects them it stimulated the hypothalamus to induce the thermostat in the brain to get rid of the body of the pyrogens or to reduce the pyrogens influx in the body (Mota-Rojar et al., 2021). Pyrexia possibly also could be associated with viraemia, host response to dead cells and interleukin-1 (IL-1)-mediated elevation of the hypothalamus induced by IBDV (Cho and Edgar, 1972; El-Radhi, 2018). The absence of pyrexia in group E1, which were not inoculated with IBDV could support the earlier deduction. The vaccinated groups C1, C2, C3 and F produced immunoglobulins which neutralized the Gumboro antigens hence they did not experience pyrexia when compared to the groups not vaccinated but inoculated with vvIBDV. Groups A, E1, E2 and E3 had normal cloacal temperatures because they were biosecured and not inoculated with vvIBDV. In group B, cloacal temperature did increase 3 dpi because there was no anamnestic response. Cloacal temperatures were similar and normal at 4 dpi because inflammatory cells were able to destroy the IBDV that caused pyrexia in the chickens. The clinical implication of the findings is that biosecurity might have played an important role in maintaining normal cloacal temperature in groups A, E1, E2 and E3 and vaccination and nutraceuticals also played part in suppressing rise in cloacal temperature in groups C1, C2 and C3. Pullets in the unvaccinated groups D1 and D3 had high cloacal temperature probably because of lack of vaccination and their bodies did not produce immunoglobulins.
Morbidity rate in groups A, E1, E2, and E3 was not recorded because they were not inoculated with vvIBDV and also in groups C1, C2 and C3 as they were administered nutraceuticals and vaccinated with Gumboro vaccine. Morbidity rate was recorded in group B because it was inoculated with vvIBDV without administration of nutraceuticals, and vaccinated. Morbidity rates due to IBD have been reported in chickens by several researchers (Congrove, 1962; Abdu et al., 1987; Orakpoghenor et al., 2020). Groups D1, D2 and D3 had the highest morbidity rates probably because the administered nutraceuticals alone (King herb oral solution oral solution, Gumbo ND and Grand humi vet) could not modulate prompt antibody response following inoculation with vvIBDV. At 3 dpi the lowest morbidity rate was observed in the group (D2) that was not vaccinated but administered Gumbo ND and inoculated with vvIBDV. At 3 dpi mortality was not observed in groups A, E1, E2, and E3 because the pullets were not inoculated with vvIBDV, similarly there was no mortality in groups C1, C2, C3 and F because they were vaccinated against vvIBDV. Mortality was observed in groups D1 and D3 because they were inoculated with vvIBDV and not vaccinated.
Nutraceuticals improved feed efficiency since the contents have nutritive values. This was particularly evident in group C1 which was given King herb oral solution with the highest live body weight gained, while group B had the lowest live body weight gained because no nutraceutical was administered. The live body weight of birds decreased in groups B, D1, D2, D3 and F due to vvIBDV inoculation and increased in groups A, C1, C2, C3, E1, E2, and E3. It has been reported that vvIBDV decrease live body weight as a result of anorexia (Landy et al., 2020). The clinical implication is that the presence of vvIBDV infection causes decrease in live body weight. This further elucidates that biosecurity is the best method to control Gumboro disease, couple with nutraceuticals and vaccination.
The gross lesions observed in this study especially in the BF, was as previously reported in several IBD outbreaks (Aliyu et al., 2016, Zeryehun et al., 2012; de Wit and Baxendale, 2013 and Orakpoghenor et al., 2020). The dehydration and emaciation due to IBD in pullets in this study could be attributed to loss of fluid from diarrhoea and decreased feed and water intake resulting from anorexia. Diarrhoea resulted from inflammatory processes triggered by the IBDV in gut-associated lymphoid tissues leading to intestinal secretion disruption (Abaidullah et al., 2019 and Orakpoghenor et al., 2020). Haemorrhages in the pectoral, thigh and leg muscles and BF in IBD might be associated with virus-induced deficiency in the coagulation cascade resulting from disseminated intravascular coagulopathy, endothelial cells damage, prolonged whole blood recalcification, prothrombin time, activated partial thromboplastin time or a combination of these changes (Zeryehun et al., 2012). The IBDV target primarily the IgM B-cells of the BF (Shah et al., 2021). The gross lesions observed in the BF lead to inflammatory reaction accompanied by release of cytokines (Rauw et al., 2007).
Absence of histopathology lesions in group A and E1 was not expected because the bird in the groups was not inoculated with IBDV. At 3 dpi, however, groups B and D1, D2 and D3 had lesions in the BF, which included; interfollicular oedema, widen interfollicular spaces, depletions of lymphocytes of follicular cortices and medullae, heterophilic infiltration of follicular cortices, vacuolations and cystic formations. In groups C2, C3 and F there were widen interfollicular spaces, depletion of lymphocytes in the follicular cortex and medulla in the BF. The above histopathological changes were due to the tissue damage by vvIBDV in lymphoid tissues even in the presence of nutraceuticals and vaccination. Infact, previous studies have shown that some remedies used by poultry farmers induce histopathological changes in FB of affected birds (Shallmizhili et al., 2018).
Conclusion
The following conclusions were made from the findings of this study: Clinical signs (%) were not observed in pullets administered the three nutraceuticals, vaccinated with Gumboro vaccine and inoculated with vvIBDV while 28.67 of those that did not receive nutraceuticals but inoculated with vvIBDV showed clinical signs at 3 dpi. At 3 dpi the cloacal temperature (°C) of pullets administered Grand humiVet (40.98 ± 0.09) and Gumbo ND (41.12 ± 0.08), vaccinated with Gumboro vaccine and inoculated with vvIBDV were significantly lower (P ≤ 0.05) than those that did not receive nutraceuticals but inoculated (42.30 ± 0.19). At 4 dpi morbidity and mortality rates (%) were not recorded in pullets administered the three nutraceuticals, vaccinated with Gumboro vaccine and inoculated with vvIBDV while the rates were 25.00/5.88 in those that did not receive nutraceuticals but inoculated. At 3 dpi the live body weights (g) of pullets administered King-herb oral solution (367.30 ± 12.28), Gumbo ND (346.10 ± 9.48) or Grand humiVet (327.00 ± 5.18), vaccinated with Gumboro vaccine and inoculated with vvIBDV were significantly higher (P ≤ 0.05) compared to those that did not receive the nutraceuticals but were inoculated (253.60 ± 12.25). At 3 dpi the percentage gross lesion (%) of pullets administered King-herb oral solution (2.50), Gumbo ND (5.83) or Grand humiVet (4.58), vaccinated with Gumboro vaccine and inoculated with vvIBDV was lower (P ≤ 0.05) than the value of 38.08 recorded in those that did not receive nutraceuticals but were inoculated. At 3 dpi the mean histopathological score of pullets administered King-herb oral solution (3.45 ± 0.05) or Grand humiVet (3.63 ± 0.13), vaccinated with Gumboro vaccine and inoculated with vvIBDV were significantly lower (P ≤ 0.05) compared to those that did not receive nutraceutical but inoculated (4.50 ± 0.17). Farmers could administer nutraceuticals at 5 to 24 days of age and vaccinate against IBD at 11 and 22 days of age orally to pullets in order to prevent disease caused by vvIBDV. Farmers could administer nutraceuticals at 5 to 24 days of age and vaccinate against IBD at 11 and 22 days of age orally to pullets to improve body weight gain.
References
- Abaidullah, M., Peng, S., Kamran, M., Song, X. and Yin, Z. (2019). Current findings on gut microbiota mediated immune modulation against viral diseases in chicken. Viruses, 11(8): 681-682.
- Abdu, P. A. (1987). A retrospective study of infectious bursal disease. In: Proceeding of the 11 th Annual Conference of the Nigerian Society for Animal Production, Zaria, Pp. 65-69
- Abdu, P.A. (1988). Case report: Infectious bursal disease in a flock of broilers and local chickens in Nigeria. Bulletin of Animal Health and Production in Africa, 36: 269-271.
- Abdu, P.A. (2007). Viral diseases. In: Manual of Important Poultry Diseases in Nigeria. 2nd edn.MacChin Multimedia Designer, Zaria, Pp. 15-24.
- Abdu, P.A. and Musa, U. (2014). Infectious bursal diseae. In: Textbook of Avian Medicine First Edition. Ndahi, P. A. Printing, S/Gari Zaria, Nigeria, Pp. 92-94.
- Abdul, R., Murgia, M. V., Rodriguez-Palacios, A., Lee, C. W. and Saif, Y. M. (2013). Persistence and tissue distribution of infectious bursal disease virus in experimentally infected SPF and commercial broiler chickens. Avian Disease case in free-range chickens. Avian Diseases, 57: 759-766.
- Aliyu, H.B., Sa’idu, L., Jamilu, A., Andamin, A.D. and Akpavie, S.O. (2016). Outbreaks of virulent infectious bursal disease in flocks of battery cage brooding system of commercial chickens. Journal of Veterinary Medicine Volume, 7.
- Alkie, T. N., and Rautenschlein, S. (2016). Infectious bursal disease virus in poultry: current status and future prospects. Veterinary Medicine: Research and Reports, 7: 9-18.
- Babiker, M. A. A. and Tawfeeg, E. (2008). Role of administration of anti-infectious bursal disease virus (Gumboro) vaccine on immunization of chicken. International Journal of Poultry Science, 7(3): 279-282.
- Babiker, M.A.A., Yahia, I.E., Noura, K. and Manal, M.E. (2008). Evaluation of four commercial anti-infectious bursal disease vaccines under Sudan condition. International Journal of Poultry Science, 7(6): 560-573.
- Bennoune, O. (2011). The effect of bursa of Fabricius on the bacterial activity of heterophils in broiler chicken (Doctoral dissertation, thesis for graduation PhD, University of Batna, Algeria).
- Besseboua, O., Benbarek, H. and Ayad, A. (2015). Determination of the optimal time of vaccination against infectious bursal disease virus (Gumboro) in Algeria. Onderstepoort Journal of Veterinary Research, 82(1): 1-6.
- Cardoso, T. C., Rosa, A. C. G., Astolphi, R. D., Vincente, R. M., Novais, J. B., Hirata, K. Y. and Luvizotto, M. C. R. (2008). Direct detection of infectious bursal disease virus from clinical samples by in situ reverse transcriptase-linked polymerase chain reaction. Avian Pathology, 37(4): 457-461.
- Cereno, T.N. (2013). Infectious bursal disease (IBD), causative agent, diagnosis and prevention. Retrieved May 2, 2013 from www.canadianpoultry.com.
- Cheville, N. F. (1967). Studies on the pathogenesis of Gumboro disease in the bursa of Fabricius, spleen, and thymus of the chicken. The American Journal of Pathology, 51(4): 527-530.
- Cho, Y. and Edgar, S. A. (1972). Characterization of infectious bursal disease. Poultry Science, 51: 60-69.
- Cosgrove, A. S. (1962). An apparently new disease of chickens-avian nephrosis. Avian Diseases 6: 385–389.
- de Wit, J.J. and Baxendale, W. (2013). Gumboro. Retrieved on 3/07/2015, 11:56 pm from www.gumboro.com.
- Dey, S., Pathak, D. C., Ramamurthy, N., Maity, H. K. and Chellappa, M. M. (2019). Infectious bursal disease virus in chickens: prevalence, impact, and management strategies. Veterinary Medicine: Research and Reports, 10: 85-90.
- Durojaiye, O.A., Ajibade, H.A. and Olafimihan, G.O. (1984). An outbreak of infectious bursal disease in 20 weeks old birds. Tropical Veterinarian, 2: 175-176.
- El-Radhi, A. S. (2018). Pathogenesis of fever. In: El-Radhi, A. S. (ed). Clinical Manual of Fever in Children. Springer, Cham, Switzerland.
- Ertekin, A., Y?ld?r?m, B. A., Y?ld?r?m, S., Y?ld?r?m, F. and Tütüncü, M. (2016). Investigation of the lipid peroxidation, antioxidant enzymes, antioxidant vitamins, oxidation products of nitric oxide and some biochemical parameters in chicken with infectious bursal disease (IBD). European Poultry Science/Archiv für Geflügelkunde, 80(164).
- Farooq, M., Durrani, F. R., Imran, N., Durrani, Z., and Chand, N. (2003). Prevalence and economic losses due to infectious bursal disease in broilers in Mirpur and Kotli districts of Kashmir. International Journal of Poultry Science, 2: 267-270.
- Gana, B. A., Yidawe, P. J., Johnson, S., Bassey, E. A., and Ayuba, A. P. (2019). Antibody response to Newcastle disease vaccine of cockerels challenged with virulent infectious bursal disease virus and administered some complementary and alternative therapies. Journal of Applied Sciences, 19(5): 466-472.
- Hinks, A., Jacob, K., Mashouri, P., Medak, K. D., Franchi, M. V., Wright, D. C., and Power, G. A. (2022). An increase in serial sarcomere number induced via weighted downhill running improves work loop performance in the rat soleus. bioRxiv, 2022-02.
- Igwe, A. O. (2017). Comparative study of genetic influence on the susceptibility of exotic cockerels, pullets and broilers to infectious bursal disease virus. Nigerian Veterinary Journal, 38(3): 235-249.
- Ileojo, M.P. (2004). A New Geography of Nigeria. Published by Longman Nigeria PLC 52 Oba Akran, Ikeja, Lagos, Nigeria.
- Joseph, G. (2019). Evaluation of the prophylactic ability of Chick-on®, King herbs®, Vitalyte extra®, antimicrobial cocktail and Khaya senegalensis leaves on infectious bursal disease in broiler chickens.MSc Thesis. Department of Veterinary Medicine, Faculty of Veterinary Medicine, Ahmadu Bello University, Zaria, Nigeria. Pp. 50-85.
- Khan, R. S. A., Sajid, S., Habib, M., Ali, W., Shah, M. S. U. D. and Sarfraz, M. (2017). History of Gumboro (infectious bursal disease) in Pakistan. Saudi Pharmaceutical Journal, 25(4): 453-459.
- Khenenou, T., Bougherara, M., Melizi, M. and Lamraoui, R. (2017). Histomorphological study of the bursae of Fabricius of broiler chickens during Gumboro disease in Algeria area. Global Vet, 18(2): 132-136.
- Landy, N., Kheiri, F., and Faghani, M. (2020). Evaluation of cottonseed bioactive peptides on growth performance, carcase traits, immunity, total antioxidant activity of serum and intestinal morphology in broiler chickens. Italian Journal of Animal Science, 19(1): 1375-1386.
- Ley, D.H., Yamamoto, K. and Bickford, A.A. (1983). The pathogenesis of infectious bursal disease: serologic, histopathologic, and clinical chemical observations. Avian Diseases, 27: 1060-1085.
- Lukert, P.D. and Saif, Y.M. (1997). Infectious bursal disease. In: Diseases of Poultry. 10th Eds. Calnex, B.W., Baenes, H.J., Beard, C.W., McDaugald, L.R and Saif, Y.M. (Eds). Iowa State University Press, Ames, Iowa, USA, Pp. 721-738.
- Mota-Rojas, D., Wang, D., Titto, C. G., Gómez-Prado, J., Carvajal-de la Fuente, V., Ghezzi, M., and Martínez-Burnes, J. (2021). Pathophysiology of fever and application of infrared thermography (IRT) in the detection of sick domestic animals: Recent advances. Animals, 11(8): 2316-2320.
- Musa, I. W., Saidu, L., and Abalaka, E. S. (2012). Economic impact of recurrent outbreaks of gumboro disease in a commercial poultry farm in Kano, Nigeria. Asian Journal of Poultry Science, 6(4): 152-159.
- Nawathe, D.R., Onunkwo, O. and Smith, I.M. (1978). Serological evidence of infectious with the virus of infectious bursal disease in wild and domestic birds in Nigeria. Veterinary Records, Pp. 102-144.
- Nunoya, T., Otaki, Y., Tajima, M., Hiraga, M. and Saito, T. (1992). Occurrence of acute infectious bursal disease with high mortality in Japan and pathogenicity of field isolates in specific-pathogen-free chickens. Avian Diseases, 36: 597-609.
- Office International des Epizooties (OIE) (2004). Manual of Diagnosis Tests and Vaccines for Terrestrial Animals.5th edn. Chapter 2-7-1, part 2, section 2-7.
- Orakpoghenor, O., Oladele, S. B. and Abdu, P. A. (2020). Infectious bursal disease: transmission, pathogenesis, pathology and prevention – an overview. World Poultry Science Journal, 76(2): 292-303.
- Orakpoghenor, O., Oladele, S. B., Abdu, P. A., Markus, T. P., Enam, S. J., Andamin, A. D., and Esievo, K. A. N. (2021). Pigeons (Columba livia domestica) are susceptible to infectious bursal disease: A Comparative Study of their hematological and serum biochemical alterations. Frontiers in Veterinary Science, 8: 673398.
- Rauw, F., Lambrecht, B. and van den Berg, T. (2007). Pivotal role of ChIFNγ in the pathogenesis and immunosuppression of infectious bursal disease. Avian Pathology, 36(5): 367-374.
- Shah, A. U., Li, Y., Ouyang, W., Wang, Z., Zuo, J., Shi, S. and Yang, Q. (2021). From nasal to basal: single-cell sequencing of the bursa of Fabricius highlights the IBDV infection mechanism in chickens. Cell and Bioscience, 11(1): 1-24.
- Shallmizhili, J.J (2018). Evaluating the efficacy of some unconventional remedies used for the control of infectious bursal disease in Nigeria. MSc Thesis. Department of Veterinary Medicine, Faculty of Veterinary Medicine, Ahmadu Bello University, Zaria, Nigeria. Pp. 50-85.
- Williams, A. E and Davison, T. F. (2005). Enhanced immunopathology induced by very virulent infectious bursal disease virus. Avian Pathology, 34: 4-14.
- Wyeth, P. J. and Cullen, G. A. (1976). Maternally derived antibody?effect on susceptibility of chicks to infectious bursal disease. Avian Pathology, 5(4): 253-260.
- Zeryehun, T., Hair-Bejo, M. and Rasedee, A. (2012). Hemorrhagic and clotting abnormalities in infectious bursal disease in specific-pathogen-free chicks. World Applied Sciences Journal, 16(98): 1123-1130.
Citation: Joseph Godwin, Abdu Paul Ayuba, Wakawa Aliyu Mohammed and Oladele Sunday Blessing. (2024). Prophylactic Effects of Three Nutraceuticals Administered Singly or with Intermediate Gumboro Vaccine on Clinicopathological Changes in Pullets Experimentally Infected with Gumboro Virus. Archives of Veterinary and Animal Sciences 6(1).
Copyright: © 2024 Joseph Godwin. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

