Research Article
Volume 4 Issue 1 - 2022
Effect of Trypanosoma brucei brucei and Trypanosoma evansi on Ascorbic Acid and Semen Quality and its Relation with Free Testosterone in Yankasa Rams.
Department of Zoology, Adamawa State University, Mubi- Nigeria.
*Corresponding Author: Elihu A, Department of Zoology, Adamawa State University, Mubi- Nigeria.
Received: March 03, 2022; Published: March 15, 2022
Abstract
Infection. The effect being severe in T. b. brucei but moderate in T. evansi infection and mixed infections which may affect spermatogenesis and male secondary sex characteristics. Seminal plasma ascorbic acid may have considerable role in improving male fertility potential in the control group. Ascorbic acid is present at approximately ten fold higher concentration in seminal plasma as compared to blood and is mostly secreted from seminal vesicles. Depletion of ascorbic acid pool of the host during the infection was postulated as a possible cause of the iron sequestration. Oxidative stress is known to be implicated in a variety of pathophysiological events that cause male infertility. The present study aimed to investigate the effect of T. evansi and T. b. brucei onseminal plasma ascorbic acid and semen quality and its relation to serum free testosterone in Yankasa rams. Sixteen (16) Yankasa rams aged between 24 and 30 months and weighed between 22 to 25 kg were .purchased from Tike market Mubi. They were screened for the presence of endo and ectoparasites. The rams were thereafter treated with Oxytetracycline (Tridax®) intramuscularly, at a dose of 20 mg/kg. Body weight and Albendazole orally, at a dose of 7.5 mg/kg body weight. The rams were sprayed against ectoparasites with Diazinon (Diaznol®, Animal Care, Nig. Ltd.). They were allowed to acclimatized for four weeks and ear-tagged for the purpose of identification in a clean fly proof house, adequately fed and given water ad libitum. By the end of the four weeks acclimatization the rams were randomly grouped into four experimental groups of four rams each, based on their weights. The rams in groups 1, 2 and 3 were experimentally infected with T. brucei brucei, (Emodike strain), T. evansi and mixed inoculum of both parasites, respectively while those in group 4 served as the naive control. Each infected ram received 2ml containing 2x106 trypanosomes via the jugular vein. Each infected ram received 2ml containing 2x106 trypanosomes via the jugular vein. The seminal plasma ascorbic acid was measured spectrophotometrically. Ascorbic acid stock solutions were prepared from L ascorbic acid. The HPLC grade methanol and acetic acid glacial were used. 0.45 µm Millipore solvent filter, was used for all reagent and standard solutions. All samples were vortexed for 20 seconds and cold-centrifuged at 10000 rpm for 3 min. Each supernatant was kept at −24°C until a 20 µl aliquot was injected onto the HPLC system (Buck scientific BLC 10/11, Las Vegas, USA). Blood was obtained through vein puncture into vaccutainer tubes for serum testosterone analysis. It was then centrifuged at 1500rpm for 5 min and serum obtained was stored at -200C until laboratory analysis was carried out. Serum testosterone was determined using testosterone enzyme immunosorbent assay (ELISA) kit (Diagnostic Automation/Cortes Diagnostic Inc. Immuno Diagnostics CA, Woodland Hills, Califonia) according to the manufacturer’s instructions. Each sample was analyzed in dublicate and absorbance was measured at 450 nm with an ELISA reader (ROBONIK ELISA Plate Analyser, model: Readwell Touch). The seminal plasma ascorbic acid in this study was significantly lower in the infected rams than in the normal control rams. In this study there was increase (higher) semen volume in the infected compare to the control group which could be due to high genetic potential and better semen production. The semen concentration of the infected group was significantly lower compared to the uninfected control animals. Examination of testosterone profile of the infected Yankasa rams revealed that these animals suffered from a significant decrease in the levels of testosterone when compared to the control group the present study concluded that the testosterone production in Yankasa rams were significantly reduced or depleted following trypanosome
Keywords: T. b. brucei; T. evansi; Ascorbic acid; Semen quality; Testosterone
Introduction
Trypanosomiasis is well known as one of the leading causes of limiting livestock production in Africa, especially in the sub-Saharan region. From the economic point of view, it is one of the main factors which threaten the food security in Africa especially in a country like Nigeria (Amin et al., 2020). T.brucei brucei has no regional epizootical limitations and it causes virulent disease described as Nagana in animals side by side with sleeping sickness in several part of sub-saharan Africa. Sheep infected with T. brucei brucei developed acute coagulative necrosis and leucocytes infiltration of the anterior pituitary as well as localization of trypanosomes in the pituitary tissue. T. evansi causes an epidemic of a disease called “Surra”, which is of great economic importance in Africa. The mammalian testis fulfils a dual role of exocrine and endocrine organ by producing spermatozoa and testosterone respectively. Conventional semen parameters, mainly sperm count, motility and morphology are among the many predictive factors for male infertility (Agarwal et al., 2016). A considerable amount of variability has been shown to exist in various semen characteristics within and among individuals. These variations have been largely attributed to several modifiable intrinsic and extrinsic factors. These factors include the length of sexual abstinence, ejaculation frequency and method of collection. Other factors that have the potential to influence semen quality are general health and lifestyle, infection, dysfunction of male sex glands (Ayad et al., 2018). Seminal plasma is a complex fluid, which serves as a vehicle for transporting ejaculated spermatozoa towards their journey from the testes to their target, the oocyte. Seminal plasma not only transports the spermatozoa but also provides protection and nutrition to the spermatozoa during their onward movement in the female reproductive tract (Talluri et al., 2017)
Ascorbic acid is present at approximately ten fold higher concentration in seminal plasma as compared to blood and is mostly secreted from seminal vesicles. Ascorbic acid acts as an electron donor that reacts with superoxide, hydroxyl and peroxide radicals to form dehydroascorbic acid. Sperm cytoplasmic volume is very low and its cytoplasm contains only low concentrations of free radical scavenging enzymes. In contrast, seminal plasma is well endowed with an array of antioxidant defence mechanism to protect spermatozoa against oxidants (Kothari et al., 2017). Ascorbic acid level in seminal plasma were found to be positively correlated with sperm concentration, sperm motility, normal sperm morphology and closely related to male fertility (Ko et al., 2012). Oxidative stress is an imbalance between free radical generating and scavenging activities, resulting in the accumulation of oxidation products which are capable of causing tissue damage (Al-saab, 2015). Depletion of ascorbic acid pool of the host during the infection was postulated as a possible cause of the iron sequestration (Igbokwe, 2018). Oxidative stress is known to be implicated in a variety of pathophysiological events that cause male infertility (Fraczek et al., 2020).
Testosterone is responsible for the masculinization characteristics and development of secondary male sex characteristics. Testosterone’s function during puberty includes development of the penis, enlargement of the prostate and seminal vesicles, increasing bone mineral density, and redistribution of body fat and hair (Wilczynski and Lily Agrawal, 2015). Testosterone is a key mediator in the expression of numerous morphological and behavioral traits in mammals, but the factors underlying individual variation in circulating testosterone levels are poorly understood. The level of testosterone in the blood circulation of rams varies by breed, age, nutrition, season, and manifestations of estrus in ewes (Maksimovic et al., 2016).
The present study aimed to investigate the effect of T. evansi and T. b. brucei on seminal plasma ascorbic acid and semen quality and its relation to serum free testosterone in Yankasa rams.
Materials and Method
Sixteen (16) Yankasa rams aged between 24 and 30 months and weighed between 22 to 25 kg were .purchased from Tike market Mubi. They were screened for the presence of endo and ectoparasites.The rams were thereafter treated with Oxytetracycline (Tridax®) intramuscularly, at a dose of 20 mg/kg.body weight and Albendazole orally, at a dose of 7.5 mg/kg body weight. The rams were sprayed against ectoparasites with Diazinon (Diaznol®, Animal Care, Nig. Ltd.). They were allowed to acclimatized for four weeks and ear-tagged for the purpose of identification in a clean fly proof house, adequately fed and given water ad libitum. By the end of the four weeks acclimatization the rams were randomly grouped into four experimental groups of four rams each, based on their weights. Group 4 (n = 4) represent uninfected control group and group 1 (T .b. brucei) and group 2 (T. evansi and group 3 (mixed infection) (n = 4 each) represent infected groups. Each infected ram received 2ml containing 2x106 trypanosomes via the jugular vein.
Semen collection
Semen collection was done weekly between 9am and 10am, by electrostimulation with the help of a portable battery-powered electro-ejaculatory mini tube (Lane Ram Ejaculator, model C27113) for small ruminants. The rams were adequately restrained, the prepuce was washed and dried using cotton wool soaked in diluted chloroxylenol (0.002%; DettolR) to remove dirts and debris. The probe of the electro-ejaculator was lubricated using petroleum jelly and inserted into the animal‘s rectum and switched on, this result in erection and subsequently, ejaculation. Semen begin to flow once the animal has achieved excitation by the stimulatory action of the electroejaculatory device. The impulses consist of applying the stimulus at an interval of 5 seconds, with 5 seconds break. The ejaculates was collected into pre-warmed, sterile and graduated transparent collection tube, labelled and kept in a water bath at a temperature range of 35-37ºC. This was done to prevent temperature changes which may affect the quality of semen before analysis. Seminal parameters (volume, pH, sperm concentration, sperm motility, percent of live sperm, and percent of sperm showing morphological abnormalities) were evaluated. Semen volume was determined by direct reading the graduated tubes. pH was determined by inserting a pH meter (Jenway, model 3025) into the collection tube and the value was then taken. Sperm motility was determined by placing a drop of raw undiluted semen on a pre-warmed slide then cover-slipped and viewed using a field microscope at X40 magnification. Sperm concentration was evaluated by visual count under the microscope using improved Neubauer Haemocytometer. The sperm cells were immobilized using a 1% formaldehyde solution prior to counting. The raw semen was mixed thoroughly and filled into the unopette capillary tube with a dilution ratio of 1:10. The diluted semen was thereafter transferred unto the haemocytometer chamber and counted under the microscope. The number of sperm cells counted using the haemocytometer multiplied by a million (106) was the concentration per ml of the raw semen. The percentage live sperm and spermatozoa morphological abnormalities were determined using Eosin-Nigrosin stain technique, applied on a glass slide. The staining mixture consist of 1% Eosin B and 5% of nigrosin in 3% sodium citrate dehydrate solution. One drop of raw semen was added to one drop of the stain, thereafter it was mixed thoroughly and a fresh smear was made from it. The slide was then examined under a light binocular microscope at X40 magnification. A minimum of 100 spermatozoa was counted and the percentage of each estimated. The live-dead staining principle was based upon the observation that Eosin-B penetrate the dead sperms (thereby making them appear pink), while the viable sperm cells repelled the stain and appeared unstained (white).The seminal plasma ascorbic acid was measured spectrophotometrically and sample for serum free testosterone was sent to laboratory.
Semen collection was done weekly between 9am and 10am, by electrostimulation with the help of a portable battery-powered electro-ejaculatory mini tube (Lane Ram Ejaculator, model C27113) for small ruminants. The rams were adequately restrained, the prepuce was washed and dried using cotton wool soaked in diluted chloroxylenol (0.002%; DettolR) to remove dirts and debris. The probe of the electro-ejaculator was lubricated using petroleum jelly and inserted into the animal‘s rectum and switched on, this result in erection and subsequently, ejaculation. Semen begin to flow once the animal has achieved excitation by the stimulatory action of the electroejaculatory device. The impulses consist of applying the stimulus at an interval of 5 seconds, with 5 seconds break. The ejaculates was collected into pre-warmed, sterile and graduated transparent collection tube, labelled and kept in a water bath at a temperature range of 35-37ºC. This was done to prevent temperature changes which may affect the quality of semen before analysis. Seminal parameters (volume, pH, sperm concentration, sperm motility, percent of live sperm, and percent of sperm showing morphological abnormalities) were evaluated. Semen volume was determined by direct reading the graduated tubes. pH was determined by inserting a pH meter (Jenway, model 3025) into the collection tube and the value was then taken. Sperm motility was determined by placing a drop of raw undiluted semen on a pre-warmed slide then cover-slipped and viewed using a field microscope at X40 magnification. Sperm concentration was evaluated by visual count under the microscope using improved Neubauer Haemocytometer. The sperm cells were immobilized using a 1% formaldehyde solution prior to counting. The raw semen was mixed thoroughly and filled into the unopette capillary tube with a dilution ratio of 1:10. The diluted semen was thereafter transferred unto the haemocytometer chamber and counted under the microscope. The number of sperm cells counted using the haemocytometer multiplied by a million (106) was the concentration per ml of the raw semen. The percentage live sperm and spermatozoa morphological abnormalities were determined using Eosin-Nigrosin stain technique, applied on a glass slide. The staining mixture consist of 1% Eosin B and 5% of nigrosin in 3% sodium citrate dehydrate solution. One drop of raw semen was added to one drop of the stain, thereafter it was mixed thoroughly and a fresh smear was made from it. The slide was then examined under a light binocular microscope at X40 magnification. A minimum of 100 spermatozoa was counted and the percentage of each estimated. The live-dead staining principle was based upon the observation that Eosin-B penetrate the dead sperms (thereby making them appear pink), while the viable sperm cells repelled the stain and appeared unstained (white).The seminal plasma ascorbic acid was measured spectrophotometrically and sample for serum free testosterone was sent to laboratory.
Estimation of Ascorbic acid
Principle: Semen sample was centrifuged at 4000 rpm for 5 min. A 100 µl aliquot of supernatant was admixed with 900 µl of cold methanol (100%). All samples were vortexed for 20 seconds and cold-centrifuged at 10000 rpm for 3 min. Each supernatant was kept at −24°C until a 20 µl aliquot was injected onto the HPLC system (Buck scientific BLC 10/11, Las Vegas, USA). The experiments were performed in isocratic mode in different mobile phase composition. The mobile phase consisted of 2.5% acetic acid glacial and 80% methanol in dH2O, and then it was filtered through a 0.45 µm filter under vacuum and degassed before use. The flow rate was maintained at 1.50 ml/min and spectrophotometric detection at 254 nm was employed. The isocratic chromatographic system was conditioned by passing the eluent through the column until a stable base line was observed. Then, repeatable retention times were obtained for three subsequent injections.
Principle: Semen sample was centrifuged at 4000 rpm for 5 min. A 100 µl aliquot of supernatant was admixed with 900 µl of cold methanol (100%). All samples were vortexed for 20 seconds and cold-centrifuged at 10000 rpm for 3 min. Each supernatant was kept at −24°C until a 20 µl aliquot was injected onto the HPLC system (Buck scientific BLC 10/11, Las Vegas, USA). The experiments were performed in isocratic mode in different mobile phase composition. The mobile phase consisted of 2.5% acetic acid glacial and 80% methanol in dH2O, and then it was filtered through a 0.45 µm filter under vacuum and degassed before use. The flow rate was maintained at 1.50 ml/min and spectrophotometric detection at 254 nm was employed. The isocratic chromatographic system was conditioned by passing the eluent through the column until a stable base line was observed. Then, repeatable retention times were obtained for three subsequent injections.
Estimation of serum free testosterone
Serum testosterone was determined using testosterone enzyme immunosorbent assay (ELISA) kit (Diagnostic Automation/Cortes Diagnostic Inc. Immuno Diagnostics CA, Woodland Hills, Califonia) according to the manufacturer’s instructions. The principle of this technique is that specific antibodies to trypanosomes can be detected by enzyme-linked anti-immunoglobulins using solid-phase polystyrene plates coated with soluble antigen. 96 coated wells were secured in the reader holder and 10µl of standards, specimens and controls were dispensed into appropriate wells and 10 µl of Testosterone HRP conjugate reagent was dispensed into each well. 50 µl of rabbit anti-testosterone reagent was dispensed to each well, it was thoroughly mixed for 30 seconds and then incubated at 37ºC for 90 minutes. Microwells were rinsed and flicked 5 times with distilled water. 100 µl of TMB Reagent was dispensed into each well and was gently mixed for 10seconds. It was then incubated at room temperature (18-25ºC) for 20 minutes. Reaction was stopped by adding 100 µl of Stop solution to each well. It was gently mixed for 30 seconds. It was ensured that all the blue colour changes to yellow completely.
Serum testosterone was determined using testosterone enzyme immunosorbent assay (ELISA) kit (Diagnostic Automation/Cortes Diagnostic Inc. Immuno Diagnostics CA, Woodland Hills, Califonia) according to the manufacturer’s instructions. The principle of this technique is that specific antibodies to trypanosomes can be detected by enzyme-linked anti-immunoglobulins using solid-phase polystyrene plates coated with soluble antigen. 96 coated wells were secured in the reader holder and 10µl of standards, specimens and controls were dispensed into appropriate wells and 10 µl of Testosterone HRP conjugate reagent was dispensed into each well. 50 µl of rabbit anti-testosterone reagent was dispensed to each well, it was thoroughly mixed for 30 seconds and then incubated at 37ºC for 90 minutes. Microwells were rinsed and flicked 5 times with distilled water. 100 µl of TMB Reagent was dispensed into each well and was gently mixed for 10seconds. It was then incubated at room temperature (18-25ºC) for 20 minutes. Reaction was stopped by adding 100 µl of Stop solution to each well. It was gently mixed for 30 seconds. It was ensured that all the blue colour changes to yellow completely.
Absorbance was read at 450nm with a microliter well ELISA reader within 15 minutes. Mean absorbance was calculated for each set of reference standards, controls and samples. A standard curve was constructed by plotting the mean absorbance obtained for each reference standard against concentration in ng/ml. Mean absorbance values for each specimen was used to determine the corresponding concentration of Testosterone in ng/ml from the standard curve.
Statistical analysis
The data obtained from the study were prepared for analysis using Microsoft Office Excel (2010). Inter group comparisons of mean semen parameters was analyzed by One-Way analysis of variance (ANOVA), and Duncan Multiple Range Test (DMRT) was used to separate the means. Statistical analysis (ANOVA) was carried out using the Statistical Analysis for Sciences (SAS), version 2002.The values of P < 0.05 was considered statistically significant.
The data obtained from the study were prepared for analysis using Microsoft Office Excel (2010). Inter group comparisons of mean semen parameters was analyzed by One-Way analysis of variance (ANOVA), and Duncan Multiple Range Test (DMRT) was used to separate the means. Statistical analysis (ANOVA) was carried out using the Statistical Analysis for Sciences (SAS), version 2002.The values of P < 0.05 was considered statistically significant.
Results
There was significant difference (P<0.05) among the infected groups in ascorbic acid level. Rams T4 recorded the highest value of 3.49 mg/dl in ascorbic acid in the seminal plasma at week 1 followed by rams T2 (3.36 mg/dl) then rams T3(3.24 mg/dl) while the least was recorded in T1(3.20 mg/dl). At week 2, rams T3 recorded the highest value of (3.26 mg/dl) followed by T1 (3.03 mg/dl) then T2 (3.00 mg/dl) and the least was recorded in rams T4 (2.80 mg/dl). At week 3, T2 recorded the highest value of (2.99 mg/dl) followed by T3 (2.95 mg/dl) then T4 (2.73 mg/dl) and the least was recorded in T1 (2.68 mg/dl). At week 4, T2 recorded the highest value (3.00 mg/dl) followed by T4 (2.90 mg/dl) then T1 (2.88 mg/dl) while the least was recorded in T3 (2.83 mg/dl). At week 5 and 6, T2 recorded the highest value (3.00 and 3.27 mg/dl) followed by T4 (3.15 and 3.37 mg/dl) then T3 (3.02 and 3.35 mg/dl) while the least was recorded by T1 (2.77 and 2.84 mg/dl). At week 7, T1 recorded the least mean value of 2.23 mg/dl followed by the T4(2.56 mg/dl) then T2 (3.11 mg/dl) while the highest was recorded in T3 (3.35 mg/dl). At week 8, T3 and T4 recorded the highest value of (3.00 mg/dl each) followed by T1 (2.96 mg/dl) while the least was T2 (2.90 mg/dl). At week 9, G4 recorded highest value (3.13 mg/dl) followed by G1 (3.06 mg/dl) and the least was G2 (2.82 mg/dl) and at week 10, G4 recorded the highest value of (3.31mg/dl) followed T3 (3.18 mg/dl) then T2 (3.13 mg/dl) and the least was T1 (3.01 mg/dl). At week 11 and 12, T3 recorded the highest value (2.84 mg/dl each) followed by T1 (2.70 mg/dl each) then T2 (2.69 mg/dl each) while the least was recorded in G4 (2.56 mg/dl each)(Figure 1). Statistically significant variation was found in mean Ascorbic acid levels of the three groups (p=0.05). Seminal ascorbic acid level was found to have positive correlation with seminogram parameters like sperm concentration, motilityand normal morphology.
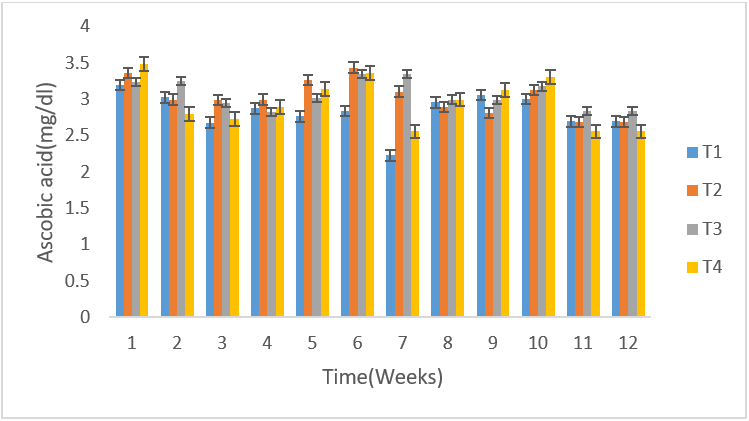
Figure 1: Mean (±SE) weekly ascorbic acid values of un-infected control rams (T4) and infected T. b. brucei rams (T1), T. evansi (T2) and mixed infection (T3).
There was no difference in the volume of semen between the infected and control group, as both fluctuated from time of infection to the termination of the experiment (Figure 2).The mean semen volumes of the infected groups were significantly higher (P < 0.05) in comparison to that of the control group. There was no significant differences (P < 0.05) in the semen pH of all the experimental groups at week 1, 2 and 3 but at week 4 T. b. brucei (T1) recorded the least semen pH value of 6.08 followed by T. evansi (T2) (7.33), mixed infection T3(8.1) and control group, T4 (8.0) it then drops in week 5 in all the groups then rose in week 6 then drops again in week 7 to 9 and continued to rise till the end of the experiment (Figure 3). The semen concentration dropped sharply in the infected group compared to the control group. However, it fluctuated in both groups till the end of the experiment (Figure 4). There was a sharp declined in sperm motility in T. b .brucei (T1) and uninfected rams (T4) from week 2 (34.48%, 47.8% respectively), week 3( 31,98% T1, 14.5% T4 respectively), week 4 (24.5% T1, 14,5% T2 and 24.5% T4 respectively while week 5 to 7 there was decline in both groups. Then it rises at week 8 in all the experimental groups (Figure 5). The mean total spermatozoa abnormalities of all the infected groups 1, 2 and 3 differed significantly (P < 0.05) from that of the control group 4 at the end of the experiment. The highest mean percent spermatozoa abnormalities were observed in T I (3.53±0.42), followed by those of T2 (2.16±0.41%) and finally T3 (1.55±0.23%). The value was lowest in the control T4 (0.18±0.08). These values were significantly increased (P<0.05) from week 1 to the end of the experiment compared to the control group, that ranged from 0.33 % -0.00% pi (figure 6).
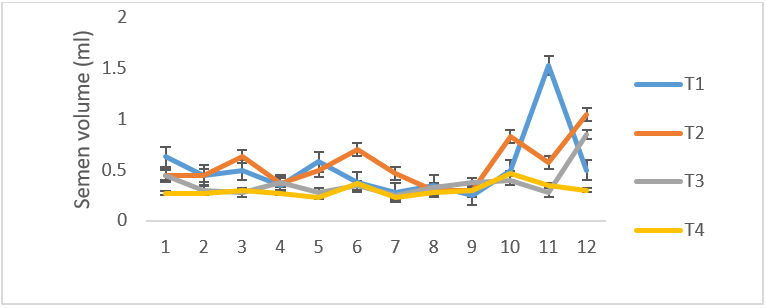
Figure 2: Mean (±SE) weekly semen volume (ml) of uninfected control Yankasa rams (T4) and rams experimentally infected with either T. b. brucei (T1), T. evansi (T2), Or both parasites (T3).
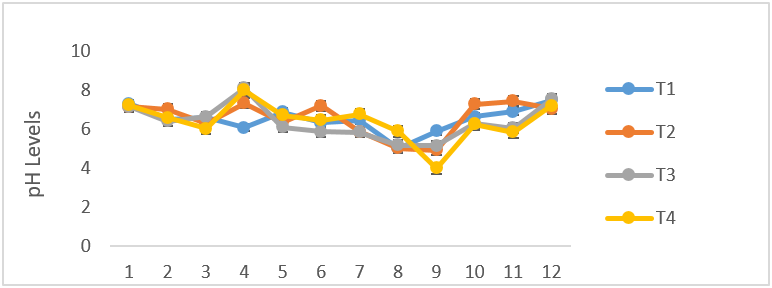
Figure 3: Mean (±SE) weekly semen pH (ml) of uninfected control Yankasa rams (T4) and rams experimentally infected with either T. b. brucei (T1), T. evansi (T2), or both parasites (T3).
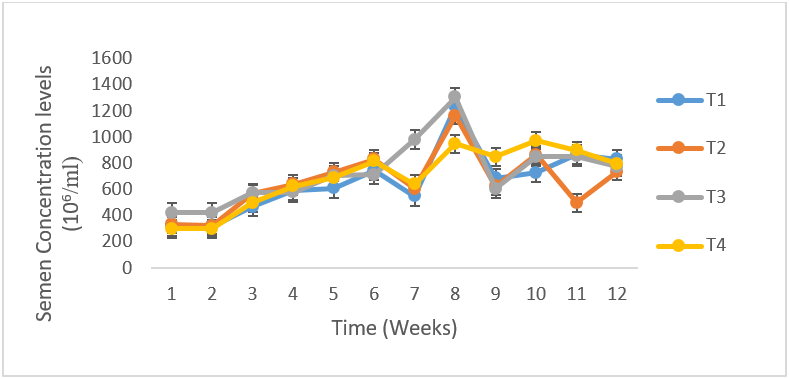
Figure 4: Mean (±SE) Sperm Concentration (106/ml) of uninfected control Yankasa rams (T4) and rams experimentally infected with either T. b. brucei (T1), T. evansi (T 2), or both parasites (T3).
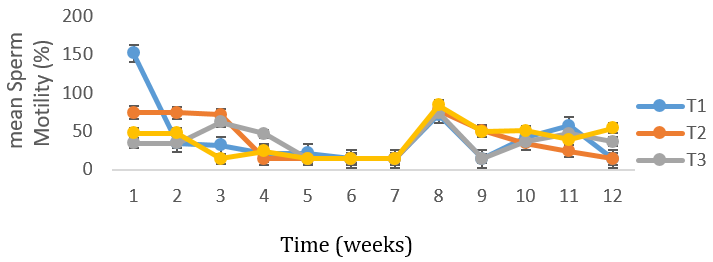
Figure 5: Mean (±SE) weekly gross forward sperm motility of uninfected control Yankasa rams (T4) and rams experimentally infected with either T. b. brucei (T1), T. evansi (T 2), or both parasites (T3).
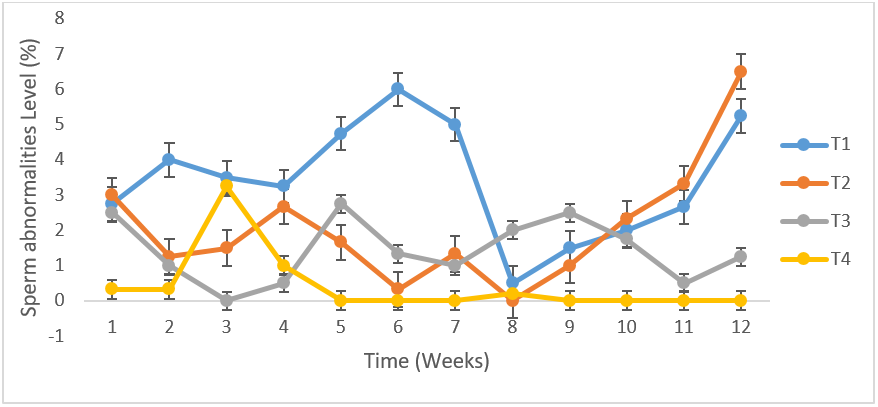
Figure 6: Total mean spermatozoa abnormalities (%) of uninfected control Yankasa rams (T4) and rams experimentally infected with either T. b. brucei (T1), T. evansi (T2), or both parasites (T3).
There were significant differences (P<0.05) among the infected groups in testosterone production. T1 rams recorded the highest value of 10.44±0.29 (ng/ml) in testosterone and this differed significantly (P<0.05) from T2 rams (8.46±0.51ng/ml). There was no significant difference (P>0.05) between T1 rams (10.44±0.29 ng/ml) and T3 (10.41±0.67ng/ml) in testosterone values. The lowest value in testosterone was observed in T2 rams (8.46±0.51ng/ml). T4 recorded higher testosterone production (10.63±0.50ng/ml) (Figure 7).
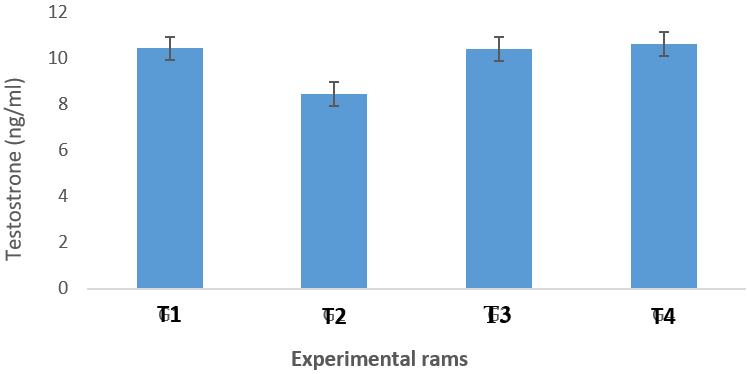
Figure 7: Testosterone profile of infected groups, T. b. brucei (T1), T. evansi (T2), or both parasites (T3) compared to the control group (T4).
Discussion
Ascorbic acid (Vitamin C) is an antioxidant for membrane compounds against free radicals generated during trypanosomiasis. There was significant difference among the treatment groups in ascorbic acid level which is in consonance with the report of Egu and Okonkwo, (2017) who reported significant differences (P < 0.05) among the treatment groups in ascorbic acid in a study on the effect of gonadotrophin (diclair®) on semen characteristics, hormonal profile and biochemical constituents of the seminal plasma of mature balami rams. The seminal plasma ascorbic acid in this study was significantly lower in the infected rams than in the normal control rams which is in agreement with the report of Kothari et al. (2017) who reported that seminal plasma ascorbic acid was significantly lower in the abnormal ejaculates than in the normal ejaculates in a study to assess the role of ascorbic acid in male fertility and its relation with free testosterone.
There was no significant difference in mean semen volumes of the infected groups in comparison to that of the control group. In this study there was increase (higher) semen volume in the infected compare to the control group which is in disagreement with the report of Wada et al. (2016b) which showed lower mean semen volumes of infected Yankasa rams and also in contrast to lower semen volume in infected Ouda rams reported by Egu (2017). But is in agreement with the report of Ogundele et al, (2016) that there was no significant difference in semen volume in the infected compare to the control rams experimentally infected with T. evansi. It is also in agreement with the report of Agarwal et al. (2016) who reported increase in semen volume in a study on the abstinence time and its impact on basic and advanced semen parameters. Increase in semen volume could be due tolength of sexual abstinence, ejaculation frequency and method of collection (Ayad et al., 2018) and also might be due to high genetic potential and better semen production.
There were no significant differences (P>0.05) among all the experimental groups in semen pH which agrees with the report of Wada et al. (2016) who reported that there was no significant difference in semen pH of all Yankasa rams and also in consonance with Li et al. (2020) who recorded a low pH of 6.96 in Chicken seminal plasma. Semen pH values obtained in this study were lower (6-6.6) than the normal range of 7-8 reported by (Egu, 2017). The measured pH depends on the length of time since ejaculation and it tends to increase shortly after ejaculation as a result of loss of CO2.
The semen concentration of the infected group was significantly lower compared to the uninfected control animals. There was high significant decrease in semen concentration in millions per mL of all the infected group when compared to the control Group. This report agrees with the work of Wada et al. (2016) who reported significant decrease in semen concentration in millions per ml of all the infected Groups of Yankasa rams experimentally infected with T. b. brucei and T. evansi and Ogundele et al. (2016) who also reported decrease in sperm concentration in Yankasa rams infected T. evansi. Among all the infected groups, semen concentration was lowest in T. evansi and was highest in Mixed infection (both T. b. brucei and T. evansi).This result is the reverse of what was been reported by Wada et al. (2016) who recorded the lowest in mixed infection and the highest in T. evansi infected rams. The decrease in semen concentration could be attributed to degeneration of seminiferous tubules, spermatic degeneration in the epididymis and reduction in number of spermatogenic cells in tubular lumen.
Mean percentage gross forward sperm (wave pattern) motility of spermatozoa in the semen was significantly lower in the control group when compared to the infected T. b. brucei and T. evansi group but significantly higher than mixed infection group. This results agrees with the report of Egu (2017) but in contrast to report of Wada et al. (2016) who reported higher forward spermatozoa motility in the control group than experimentally infected with T. b. brucei and T. evansi. The highest score in individual motility obtained in this study (43.47±5.190%) was lower than the score of 80.20% reported by Egu, (2017) in Ouda rams and 61.86±3.27 reported by Wada et al. (2016) in Yankasa rams of similar age. Sperm motility is affected by frequency of semen collection. Lower forward sperm motility in control group could be as a result of other infections.
Mean percentage gross forward sperm (wave pattern) motility of spermatozoa in the semen was significantly lower in the control group when compared to the infected T. b. brucei and T. evansi group but significantly higher than mixed infection group. This results agrees with the report of Egu (2017) but in contrast to report of Wada et al. (2016) who reported higher forward spermatozoa motility in the control group than experimentally infected with T. b. brucei and T. evansi. The highest score in individual motility obtained in this study (43.47±5.190%) was lower than the score of 80.20% reported by Egu, (2017) in Ouda rams and 61.86±3.27 reported by Wada et al. (2016) in Yankasa rams of similar age. Sperm motility is affected by frequency of semen collection. Lower forward sperm motility in control group could be as a result of other infections.
Study on the sperm morphology revealed the presence of an increase in the percentage of abnormalities (p < 0.05) in the infected animals. The significant changes were observed in the head (detached head) and tail (coil tail, bent tail) of the sperms in infected rams compared to the uninfected. This study agrees with the report of Amin et al. (2020) who reported spermatozoa abnormalities of the head and tail of dromedary bulls infected with T. evansi. The highest mean percent spermatozoa abnormalities were observed in rams infected with T. b. brucei and the lowest observed in the control group also agrees with the report of Wada et al. (2016). In the present study, the T. b. brucei -infected rams had more severe form of abnormalities followed by those with T. evansi infections. There was a significant (P<0.05) increase in the mean detached heads of the infected rams compared to the control. This result is in agreement with the report of Okubanjo et al. (2015) in T. congolense infected Yankasa rams and Ubah et al. (2017) who reported same in rams following cypermethrin treatment.
Examination of testosterone profile of the infected Yankasa rams revealed that these animals suffered from a significant decrease in the levels of testosterone when compared to the control group. These results are similar to the report of Amin et al. (2020) who showed that testosterone level decreased in dromedary bulls infected with T. evansi and also Okubanjo et al. (2015) confirmed that the experimentally infected male rat with T. congolense suffered from a reduction of testosterone, luteinizing hormone(LH) and follicle stimulating hormone (FSH) levels. The significant differences observed among the treatment group in testosterone production are in agreement with the report of Egu, (2017) who recorded significant difference among treatment group in serum testosterone production in Ouda rams. This result is also in agreement with the report of Maksimovi? et al. (2016) who reported same in Meat Institute Sheep (MIS) Serbia, Belgrade. Decrease in testosterone levels in Yankasa rams could be due to the exposure to electrical stimulation treatment and oxidative stress caused by low ascorbic acid presence in the infected rams.
Conclusion
The present study concluded that the testosterone production in Yankasa rams were significantly reduced or depleted following trypanosome infection. The effect being severe in T. b. brucei but moderate in T. evansi infection and mixed infections which may affect spermatogenesis and male secondary sex characteristics. Seminal plasma ascorbic acid may have considerable role in improving male fertility potential in the control group. Adequate seminal plasma concentration of ascorbicacid is required for normal sperm function.
Acknowledgement
The author is grateful to Mr. Joel Isa, and all staff of the Department of Animal Production Teaching and Research Farm Adamawa State University, Mubi- Nigeria for their technical assistance. Mr Ishaya Nehemiah and Mr Yahaya Babakiri of Biochemistry and Animal Production Departments and the Management of Adamawa State University, Mubi-Nigeria, is also appreciated for granting me a study fellowship (Ph.D).
The author is grateful to Mr. Joel Isa, and all staff of the Department of Animal Production Teaching and Research Farm Adamawa State University, Mubi- Nigeria for their technical assistance. Mr Ishaya Nehemiah and Mr Yahaya Babakiri of Biochemistry and Animal Production Departments and the Management of Adamawa State University, Mubi-Nigeria, is also appreciated for granting me a study fellowship (Ph.D).
References
- Agarwal, A; Esteves, S.C; Gupta, S; Sharma, R; Du- Plessis, S and Sabanegh, E (2016). Abstinence time and its impact on basic and advanced semen parameters. Journal of Urology 94(3): 059.
- Al-saab, H.K.J (2015). Effect of vitamin C injection on semen quality of Awassa rams. Diyala Agricultural Sciences Journal, 7(2): 12-19.
- Amin, Y.A, Noseer E.A, Fouad,S.S, Ali R.A, Mahmoud H.Y.A.H. (2020).Changes of reproductive indices of the testis due to Trypanosoma evansi infection in dromedary bulls (Camelus dromedarius) Semen picture hormonalprofile, histopathology, oxidative parameters, and hematobiochemical profile. Journal Advance Vetinary .Animal Research 2020; 7(3): 537–545.
- Ayad, B.M; Van der Horst, G; Du Plessis, S.S (2018). Revisiting the relationship between the ejaculatory abstinence period and semen characteristics. International Journal of Fertility and Sterility. 11(4): 238-246.
- Egu U.N (2017). Effect of Gonadotrophin (Pergonal®) on Semen Characteristics, Hormonal Profile and Biochemical Constituents of the Seminal Plasma of Mature Ouda Rams. Dairy and Vetinary Science Journal. 2(5): 555598.
- Egu, U.N and Okonkwo, J.C (2017). Effect Of Gonadotrophin (Diclair®) On Semen Characteristics, Hormonal Profile And Biochemical Constituents Of The Seminal Plasma Of Mature Balami Rams. Banat Journal of Biotechnology, VIII (15): 90-97.
- Fraczek M , Wojnar L, Kamieniczna M, Piasecka M , Gill K, Kups M et al. (2020). Seminal Plasma Analysis of Oxidative Stress in Di?erent Genitourinary Topographical Regions Involved in Reproductive Tract Disorders Associated with Genital Heat Stress. International Journal of Molecular Science. 21: 6427.
- Igbokwe I.O (2018). Evolving anti-disease strategies from biochemical pathogenesis of African trypanosomiasis. Advanced Cytology and Pathology 3(2): 33–39.
- Kothari R, Waghmare S and Choundhari A.R (2017). Role of Ascorbic Acid in Male Fertility and its Relation with Free Testosterone. International Journal of Comtemporary Medical Research. 4(6):77-83.
- Ko E. Y., S. Edmund and J. R. Sabanegh. 2012. The role of over-the-counter supplements for the treatment of male Infertility -fact or fiction? Journal of Andrology. 33: 292–308.
- Li, Y, Sun, Y, Ni, A, Shi, L, Wang, P et al. (2020). Seminal Plasma Proteome as an Indicator of Sperm Dysfunction and Low Sperm Motility in Chickens. Molecular Cell Proteomics 19(6), 1035–1046.
- Maksimovic N., Hristo, S., Stankovic, B, Mekev, C, Ruzio-Muslic, D and Caro-Petrovic, V. (2016). Investigation of serum testosterone level, scrotal circumference, body mass, semen characteristics, and their correlations in developing MIS lambs. Turkish Journal of Veterinary Animal Science. 40: 53-59.
- Ogundele F.A, Okubanjo O.O, Ajanusi O.J and Fadason S.T (2016). Effect of experimental Trypanosoma evansi infection on Haematological parameters and semen characteristic of Yankasa rams.Theriogenology, 86: 667-673.
- Okubanjo O.O., Sekoni V. O., Ajanusi O.J., Adeyeye A.A (2015). Effects of experimental Trypanosoma congolense infection on sperm morphology in Yankasa rams. Mac Veterinary Review; 38 (2): 203-208.
- Talluri T.R, Mal G, Ravi S.K. (2017). Biochemical components of seminal plasma and their correlation to the fresh seminal characteristics in Marwari stallions and Poitou jacks, Veterinary World, 10(2): 214-220.
- Wada, Y.A., Oniye, S.J, Rekwot, P.I and Okubanjo, O.O (2016b). Effect of Trypanosoma brucei brucei and Trypanosoma evansi on Scrotal Circumference, semen characteristics and haematologicsal parameters of Yankasa rams (Ovis aries Linnaeues, 1978). FUW Trends in Science & Technology Journal, www.ftstjournal.com e-ISSN: 24085162; p-ISSN: 20485170; October,1 ( 2 ): 379 – 384.
- Wilczynski C and Lily Agrawal L (2015). Testosterone Effects on the Prostate Gland: Review of Pathophysiology and considerations in Prostate Cancer. Journal of Family Medicine and Disease Prevention 1:004.
Citation: Elihu A. (2022). Effect of Trypanosoma brucei brucei and Trypanosoma evansi on Ascorbic Acid and Semen Quality and its Relation with Free Testosterone in Yankasa Rams. Archives of Veterinary and Animal Sciences 4(1).
Copyright: © 2022 Elihu A. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
