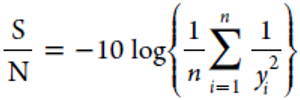Research Article
Volume 4 Issue 1 - 2022
Experimental Investigation and Feasibility Study of using Ionic Liquid for Solvent Recovery Enhancement for Extraction of Oil from the Sludge of Bottom Tank of Crude Oil
1Petroleum & Gas Engineering Department, Collage of Engineering, University of Thi-Qar, Thi Qar, Iraq
2Technical Division Officer, Industrial Water Department, Thi Qar Oil Company - Ministry of Oil, Iraq
2Technical Division Officer, Industrial Water Department, Thi Qar Oil Company - Ministry of Oil, Iraq
*Corresponding Author: Khalid Farhod Chasib, Petroleum & Gas Engineering Department, Collage of Engineering, University of Thi-Qar, Thi Qar, Iraq.
Received: December 21, 2022; Published: December 28, 2022
Abstract
The effectiveness of adding ionic liquid (IL) ([Emim][BF4]) to cyclohexane solvent to improving oil Extraction from bottom sludge of crude oil tank was investigated. A range of IL/sludge ratio (0.3−0.9 mL/g), speed of shaking (150−500 rpm), cyclohexane/sludge ratio (1−9 mL/g), and extraction duration (5−130 min) were examined. The recovery of TPH (total petroleum hydrocarbon) increased by 9.6% when addition of IL/sludge ratio equal to 0.9 mL/g (from 85.3 ± 2.3 to 94.1 ± 2.2% at 9 mL/g solvent/sludge ratio). The extraction conditions were optimizing by applied experimental design (orthogonal). Higher TPH recovery (above 96%) yielded when use of IL (i.e., IL/sludge ratio equal to 0.2 mL/g) at reduce energy consuming (95 rpm), low extraction period (8 min), and lower solvent/sludge ratio (4.3 mL/g). Compared with crude oil, the recovered oil had higher (nC16−nC34) fraction but the calorific value was similar. The results indicated that the efficient approach for treatment of oily sludge is by solvent extraction enhancement using IL with lower consumption of cyclohexane solvent.
Keywords: Extraction; Interfaces; Lipids, Sludges; Solvents; Ionic Liquids
Introduction
The petroleum industry produces a large quantity of oily sludge during its oil exploration and processing activities. [1,2] Such sludge is a complex water/oil emulsion typically including 30−50% oils, 10−20% solids, and 30−50% water by weight. [3] It also contains some aromatic hydrocarbons (e.g., benzene) and polycyclic aromatic hydrocarbons (PAHs) [4, 5]
Therefore, oily sludge has been classified as a hazardous waste in many countries. Its improper treatment can pose a serious threat to the environment and humans. [6−8] Most conventional methods for dealing with oily sludge are landfills or incineration. Because of stringent regulations in recent years, landfilling is being gradually prohibited or restricted. [1,9] Although incineration reduces a large volume of oily sludge, it requires a substantial amount of auxiliary fuel and generates a large volume of off-gases containing PAHs. [10] Recycling is an alternative option to handle oily sludge by providing the possibility for reusing valuable oil for reprocessing and recovering energy. [9,11] Solvent extraction is a commonly used approach to recycle hydrocarbons from oily waste by mixing with a desirable amount of solvents. [12] Many studies reported that a variety of variables could affect oil recovery in solvent extraction processes (e.g., temperature, pressure, solvent-to-waste ratio, mixing, and the property of solvent). [13−15] The application of solvent extraction to oily sludge treatment is desirable but challenged by its unsatisfactory extraction efficiency, large volume of solvent consumption, and long extraction duration. [11,16] Ionic liquid (IL) is a purely ionic material with a melting temperature below 100°C. It has been considered as a “green and eco-friendly solvent” because of its unique properties (e.g., thermal and chemical stability). [17-19]
A recent study by Khalid F. C. [20] found that the bitumen recovery from oil sands using IL-enhanced solvent extraction increased with the IL-to-solvent ratio and agitation time. As a result, IL can be a potentially effective candidate for enhancing the liberation of oil from solid matrices during solvent extraction. However, few studies have been reported on the use of IL in oily sludge treatment. The objective of this study was then to propose an IL-enhanced solvent extraction method for oil recovery from oily sludge. Several factors including solvent/sludge ratio, shaking speed, extraction duration, and IL/sludge ratio were examined for their impacts on oil recovery. The optimal conditions of oil recovery for two processes (i.e., solvent extraction alone and IL-enhanced solvent extraction) were also investigated.
Materials and Methods
Materials and Reagents. The oily sludge used in this study was obtained from a crude oil tank bottom in an oil refinery plant in Thi Qar Oil Company. The appearance of the oily sludge was black and sticky. Table 1 lists its characteristics. The total petroleum hydrocarbon (TPH) extraction followed the US EPA 3540C method. [20-22]. The water content was analyzed based on the ASTM D2974-00 Method A, [23-24] and the solid content was calculated according to the measured TPH and water contents. Dichloromethane (DCM) and cyclohexane used for sample extraction were of high-performance liquid chromatography (HPLC) grade (>97%, VWR International).
| Parameter | Concentration |
| TPH water contenta solid content |
58.7 ± 4% (by mass) 22.0 ± 5% (by mass) 19.3 ± 1% (by mass) |
aOn a wet-weight basis.
Table 1: Characteristics of the Oily Sludge.
Table 1: Characteristics of the Oily Sludge.
Previous studies on IL-enhanced bitumen extraction from oil sands showed that among various ILs, the use of 1-ethyl-3-methyl-imidazolium tetrafluoroborate ([Emim][BF4]) and 1-butyl-2,3-dimethyl-imidazolium tetrafluoroborate ([Bmim][BF4]) led to high-efficiency IL-free bitumen recovery. [16,18,20,22]
Therefore, [Emim][BF4] at HPLC grade (≥98%) purchased from Sigma-Aldrich was used as the IL to be investigated in this study. Silica gel (70−230 mesh, VWR international) activated at 105°C for 12 h was used to clean up the extraction solution. Anhydrous sodium sulfate (VWR international) dried at 400°C for 4 h was used to remove moisture in the extract.
Single-Factor Experiments
Single-factor experiments were designed to evaluate the influence of IL ([Emim][BF4]) addition on the oil recovery by solvent extraction with cyclohexane. Among various solvents, cyclohexane was reported to have a high bitumen recovery because it has a medium solubility (i.e., 16.8 MPa1/2) which is close to that of asphaltene (i.e., 20.1 MPa1/2). [25] Additionally, cyclohexane has a low boiling point (i.e., 80.7°C), which allows easy solvent recovery and less toxicity than many other solvents.
Single-factor experiments were designed to evaluate the influence of IL ([Emim][BF4]) addition on the oil recovery by solvent extraction with cyclohexane. Among various solvents, cyclohexane was reported to have a high bitumen recovery because it has a medium solubility (i.e., 16.8 MPa1/2) which is close to that of asphaltene (i.e., 20.1 MPa1/2). [25] Additionally, cyclohexane has a low boiling point (i.e., 80.7°C), which allows easy solvent recovery and less toxicity than many other solvents.
Therefore, cyclohexane was selected as the solvent for investigation in this study. A total of four factors were examined, including solvent/sludge (So/Sl) ratio, shaking speed, extraction duration, and IL/sludge (IL/Sl) ratio. The levels of experimental factors are presented in Table 2.
| Number | So/Sl Ratio (mL/g) | Shaking Speed (rpm) | Extraction Duration (min) | IL/Sl Ratio (mL/g) |
| 1 | 2:1, 4:1, 6:1, 8:1 | 200 | 30 | 0 |
| 2 | 2:1, 4:1, 6:1, 8:1 | 200 | 30 | 1:1 |
| 3 | 2:1 | 100, 200, 300, 400 | 30 | 0 |
| 4 | 2:1 | 100, 200, 300, 400 | 30 | 1:1 |
| 5 | 2:1 | 200 | 10, 30, 60, 120 | 0 |
| 6 | 2:1 | 200 | 10, 30, 60, 120 | 1:1 |
| 7 | 2:1 | 200 | 30 | 1:4, 2:4, 3:4, 4:4 |
Table 2: Single-Factor Experimental Conditions.
The TPH recovery rate was calculated as follows:
R = TPHr/(Co × Mo) × 100% (1)
Where R is the TPH recovery rate (%); TPHr (mg) is the mass of recovered TPH; Co (mg/g) is the TPH concentrations in the oily sludge sample (Co = 5.87 × 105 mg/kg, as listed in Table 1); and Mo (g) is the mass of the oily sludge sample.
R = TPHr/(Co × Mo) × 100% (1)
Where R is the TPH recovery rate (%); TPHr (mg) is the mass of recovered TPH; Co (mg/g) is the TPH concentrations in the oily sludge sample (Co = 5.87 × 105 mg/kg, as listed in Table 1); and Mo (g) is the mass of the oily sludge sample.
At the oil/solvent−IL interface, a black film containing suspended oil and some fine solids was formed as shown in Figure 1.
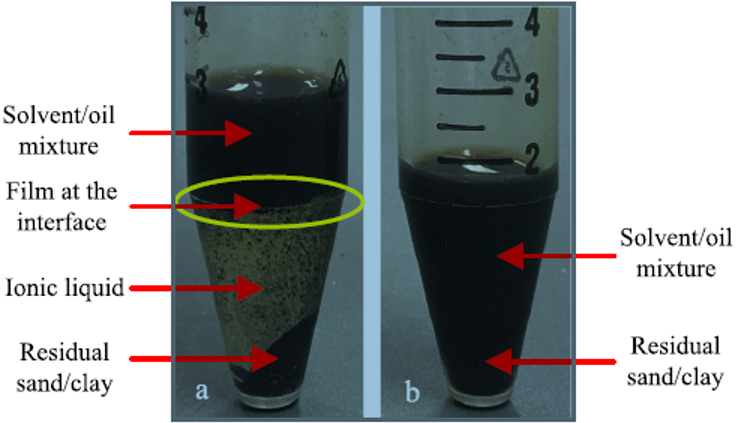
Figure 1: Phases formed by mixing (a) oily sludge with [Emim][BF4] and cyclohexane in the proportion of 1:3:2 (by weight) and (b) oily sludge with cyclohexane in the proportion of 1:2 (by weight).
Given this observation, the recovered TPH in IL-enhanced solvent extraction treatment was calculated as follows: TPHr = TPHrt + TPHri (2) where TPHr (mg) is the mass of recovered TPH; TPHrt (mg) is the mass of recovered TPH from the top oil/solvent layer; and TPHri (mg) is the mass of recovered TPH from the oil/solvent−IL interface.
Optimization of Solvent Extraction and IL-Enhanced Solvent Extraction
The optimal operational condition for each extraction process, including solvent extraction and IL-enhanced solvent extraction, was examined using orthogonal experimental design (Table 3).
The optimal operational condition for each extraction process, including solvent extraction and IL-enhanced solvent extraction, was examined using orthogonal experimental design (Table 3).
| Extraction process | Factors | Symbol | levels | ||
| 1 | 2 | 3 | |||
| solvent extraction | So/Sl ratio (mL/g) | A | 4:1 | 8:1 | 12:1 |
| shaking speed (rpm) | B | 300 | 400 | 500 | |
| extraction duration (min) | C | 30 | 60 | 120 | |
| IL-enhanced solvent extraction | So/Sl ratio (mL/g) | A | 4:5 | 7:5 | 10:5 |
| IL/Sl ratio (mL/g) | B | 1:10 | 2:10 | 3:10 | |
| shaking speed (rpm) | C | 100 | 200 | 300 | |
Table 3: Orthogonal Array Experimental Design and Factor Levels.
Signal-to-noise (S/N) ratio which combines the mean and variance was used to evaluate the experimental data. Because this study aims at maximizing the TPH recovery rate (%), the S/N ratio (dB) was calculated by the following equation:
Where yi denotes the TPH recovery observed in the ith repetition of the experimental run and n is the total number of repetitions under the same experimental condition. [21-24]
Laboratory Analysis
Chromatographic characterization of samples with petroleum hydrocarbons (PHCs) was carried out using an Agilent 6890 gas chromatograph with a flame ionization detector (GC-FID). The hydrocarbon mixtures were grouped into four PHC fractions: F1 (nC6−nC10), F2 (nC10−nC16), F3 (nC16−nC34), and F4 (nC35−nC50). Decane (nC10), hexadecane (nC16), and tetratriacontane (nC34) were used as the external standards to determine the average response factor and then quantify the PHC fractions.
Chromatographic characterization of samples with petroleum hydrocarbons (PHCs) was carried out using an Agilent 6890 gas chromatograph with a flame ionization detector (GC-FID). The hydrocarbon mixtures were grouped into four PHC fractions: F1 (nC6−nC10), F2 (nC10−nC16), F3 (nC16−nC34), and F4 (nC35−nC50). Decane (nC10), hexadecane (nC16), and tetratriacontane (nC34) were used as the external standards to determine the average response factor and then quantify the PHC fractions.
The total carbon (C), nitrogen (N), and hydrogen (H) of oil were determined using a Costech ECS 4010 elemental analyzer according to ASTM D5291-16. The Fourier transform infrared (FTIR) spectra were then obtained using a Nexus 670 Thermo Nicolet spectrometer. FTIR was used to identify if fine solids and IL exist in the RO and if the residual sand/clay contains IL after extraction.
Results and Discussion
As seen from Figure 2a−c, the extraction conditions, including solvent/sludge ratio, shaking speed, and extraction duration, had significant (P <0.05) effects on the performance of solvent extraction alone. According to Figure 2b, the TPH recovery went up gradually from 68.3 ± 3.9% at 100 rpm to 75.5 ± 1.5% at 400 rpm.
As shown in Figure 2c, the TPH recovery also experienced an upward trend with the extension of extraction duration (70.5 ± 1.3% and 76.1 ± 2.1% at 10 and 120 min, respectively).
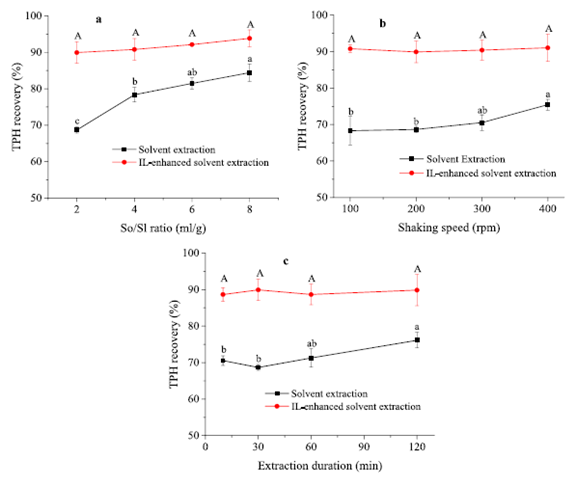
Figure 2: Influence of different operational parameters on TPH recovery: (a) So/Sl ratio (shaking speed: 200 rpm, extraction duration: 30 min, IL/Sl ratio: 1:1 mL/g), (b) shaking speed (So/Sl ratio: 2:1 mL/g, extraction duration: 30 min, IL/Sl ratio: 1:1 mL/g), and (c) extraction duration (So/Sl ratio: 2:1 mL/g, shaking speed: 200 rpm, IL/Sl ratio: 1:1 mL/g). Significant (P < 0.05) differences are shown by different letters: lowercase letter for the solvent extraction and uppercase letter for the IL-enhanced solvent extraction.
Considering the high cost of IL, a set of experiments with small IL/sludge ratios were conducted. The observed TPH recovery is shown in Figure 3. In this regard, further optimization on oil recovery was carried out using IL/sludge ratios in the range from 0.1 to 0.3 mL/g.
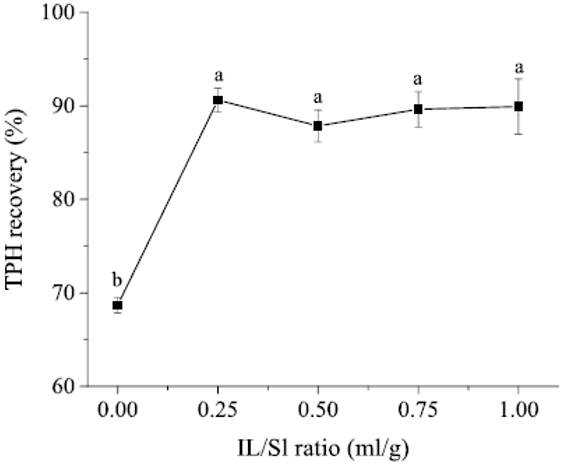
Figure 3: Influence of IL/sludge (IL/Sl) ratio on TPH recovery (So/Sl ratio: 2:1 mL/g, shaking speed: 200 rpm, extraction duration: 30 min). Different lowercase letters (a,b) indicate significant (P < 0.05) differences.
Figure 4a shows the main effects of three factors on the TPH recovery from solvent extraction alone. It can be found that the S/N ratio increased rapidly with the increasing solvent/sludge ratio (from 4:1 to 12:1 mL/g), peaking at 39.36 dB. As seen from Figure 4b−d the F4 recovery was significantly affected by all variables, whereas the solvent/sludge ratio and extraction duration were two significant factors impacting F3 recovery.
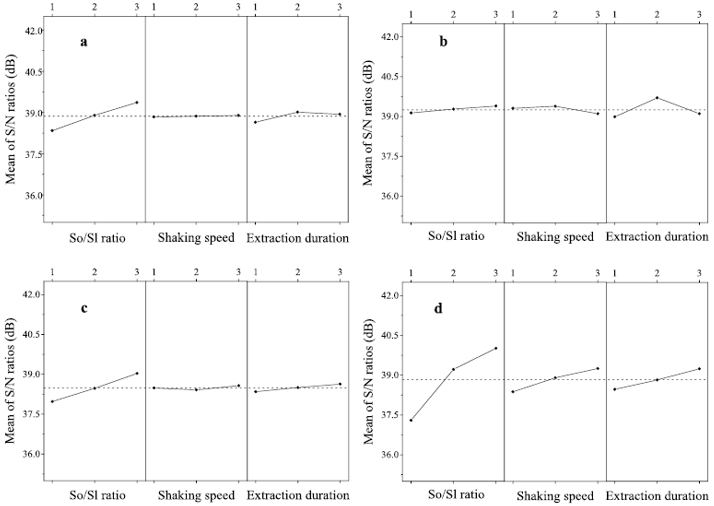
Figure 4: Effect of independent factors on (a) TPH recovery, (b) F2 recovery, (c) F3 recovery, and (d) F4 recovery of the solvent extraction alone process.
As shown in Figure 5, the S/N ratio for the TPH recovery fluctuated between 39.16 and 39.84 dB. It is interesting to note that in the IL-enhanced solvent extraction process, all variables including solvent/sludge ratio, IL/sludge ratio, and shaking speed had an insignificant influence on the TPH recovery
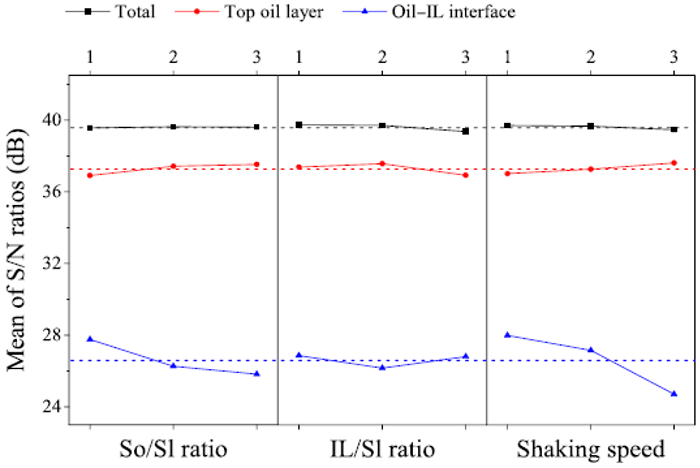
Figure 5: Effect of independent factors on the TPH recovery of the IL-enhanced solvent extraction process.
Figure 6 shows the gas chromatography (GC) chromatogram of samples from these treatments. No significant differences in composition among samples were observed because the same solvent (i.e., cyclohexane) was used in both extraction processes.
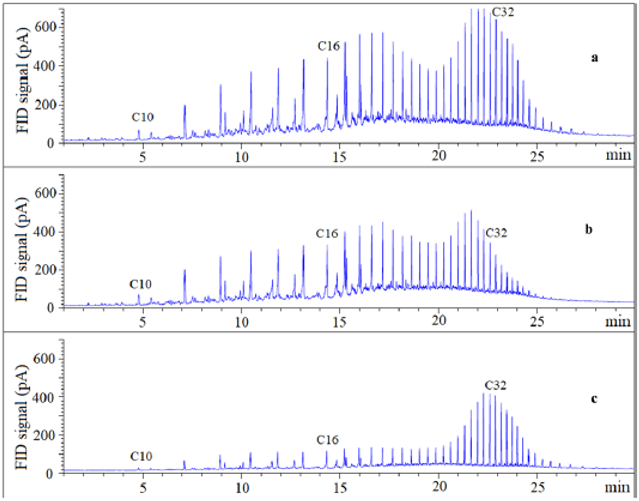
Figure 6: GC chromatogram of PHCs in (a) RO using solvent extraction alone, (b) RO from the top oil/solvent layer using ILenhanced solvent extraction, and (c) RO from the oil/solvent−IL interface using IL-enhanced solvent extraction.
As shown in Figure 7, the average F2, F3, and F4 fractions in the RO using solvent extraction alone were 37.5, 48.1, and 14.4%, respectively.

Figure 7: Distribution of PHC fractions in crude oil, RO using solvent extraction alone, RO in the top oil/solvent layer using IL-enhanced solvent extraction, and RO in the oil/solvent−IL interface using ILenhanced solvent extraction.
Conclusions
An IL-enhanced solvent extraction (using cyclohexane and [Emim][BF4]) was investigated in this study to examine its efficiency of oil recovery from oily sludge. Compared to solvent extraction alone (using cyclohexane), IL-enhanced solvent extraction achieved a similar or higher TPH recovery (96.92 ± 4.79%) within a much shorter duration (i.e., 10 min) and a much lower solvent/sludge ratio (i.e., 4:5 mL/g) under less energy consumption (i.e., 100 rpm) condition.
When compared to crude oil, the RO had a higher level of F3 fraction and similar calorific value. This indicates that oil extracts can be utilized as a potential energy source and petrochemical feedstock. After extraction, IL existed as a separate phase that can be recycled for further utilization. There was no evidence of IL and fine solids existing in the RO after IL-enhanced solvent extraction.
However, further research about the reuse of IL is still needed. In summary, IL-enhanced solvent extraction could present an economically competitive approach for the management of hazardous oily solid waste. Further work also needs to be performed to understand the extraction mechanism of the oil in the presence of IL, which will serve to the selection/design of IL for oil recovery purpose.
References
- da Silva, L. J.; Alves, F. C.; de Franc?a, F. P. (2012). A Review of the Technological Solutions for the Treatment of Oily Sludges from Petroleum Refineries. Waste Manage. Res., 30, 1016−1030.
- Khalid F. C., Basma M. K. (2021). Rectilinear Diameter for Saturated Vapor and Liquid Enthalpies at the Coexisting Phases of Pure Substances and Mixtures. Journal of Mechanical Engineering Research and Developments., 44, 351-371.
- Ramaswamy, B.; Kar, D. D.; De, S. (2007). A Study on Recovery of Oil from Sludge Containing Oil Using Froth Flotation. J. Environ. Manage., 85, 150−154.
- Overall Evaluations of Carcinogenicity: An Updating of IARC Monographs Volumes 1 to 42; IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, Ed.; World Health Organization: Lyon, France, 1987.
- Some Non-Heterocyclic Polycyclic Aromatic Hydrocarbons and Some Related Occupational Exposures; IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, Ed.; World Health Organization: Lyon, France, 2010.
- Khalid F. C., Basma M. K. (2019). Prediction of The Behavior for Polymer Blends Using Thermodynamic Model. Recent Adv. Petrochem. Sci, 6, 1−8.
- Hejazi, R. F.; Husain, T.; Khan, F. I. (2003). Landfarming operation of oily sludge in arid region-human health risk assessment. J. Hazard. Mater., 99, 287−302.
- Basma M. K. (2018). Study the Corrosion Behavior of Iron in Water Containing Chloride Ions under Controlled Conditions of Heat and Mass Transfer using Rotating Disc Electrode, Postgraduates Conference in the Iraqi Ministry of Oil, 1, 1−18.
- Khalid F. C. (2022). Chemically synthesized hydrogen fuel from reaction of methylcyclohexane over nanoporous heterogeneous catalysts Environmental Technology, 43, 1961-1967.
- Li, C.-T.; Lee, W.-J.; Mi, H.-H.; Su, C.-C. (1995). PAH Emission from the Incineration of Waste Oily Sludge and PE Plastic Mixtures. Sci. Total Environ., 170, 171−183.
- Khalid F. C. (2018). Experimental Measurement and Thermodynamic Modelling of Vapor-Liquid Equilibria Correlations for Prediction Azeotropic Behavior and Fitting Multicomponent Mixtures Data. Progress in Petrochemical Science, 1, 1−18.
- Khalid F. C. (2019). Experimental Measurement and Investigation on the Feasibility of Improvement of Physical and Dielectric Properties of Barium Titanate. Petroleum & Petrochemical Engineering Journal, 3, 1−8.
- Fisher, J. A.; Scarlett, M. J.; Stott, A. D. (1997). Accelerated Solvent Extraction: An Evaluation for Screening of Soils for Selected U.S. EPA Semivolatile Organic Priority Pollutants. Environ. Sci. Technol., 31, 1120−1127.
- Khalid F. C. (2019). Phenol Recovery from Industrial Wastewater using Various Partial – miscible organic Solvents International Journal of Modern Research in Engineering & Management (IJMREM)., 2, 1−19.
- Taiwo, E. A.; Otolorin, J. A. (2009). Oil Recovery from Petroleum Sludge by Solvent Extraction. Pet. Sci. Technol., 27, 836−844.
- Khalid F. C. (2018). Study the Vapor-Liquid Equilibrium and Excess Volume for the Binary Mixtures of Aqueous Solutions of Two Industrial Petroleum Solvents and Aromatic Hydrocarbon. Recent Advances in Petrochemical Science, 5, 1−6.
- Jose?-Alberto, M.-H.; Jorge, A. (2011). Current Knowledge and Potential Applications of Ionic Liquids in the Petroleum Industry. Ionic Liquids: Applications and Perspectives; InTech.
- Khalid F. C. (2018). Feasibility study of Improvement Experimental Measurement and Thermodynamic Modelling of Contaminants Effect on the Thermo-Mechanical Properties of Industrial Petroleum recycled plastics wastes. International Journal of Petrochemistry and Research, 2, 212-222.
- Hogshead, C. G.; Manias, E.; Williams, P.; Lupinsky, A.; Painter, P. (2011). Studies of Bitumen−Silica and Oil−Silica Interactions in Ionic Liquids. Energy Fuels, 25, 293−299.
- Khalid F. C. (2013). Extraction of Phenol from Industrial Wastewater using Organic Solvents Journal of Selcuk University Natural and Applied Science. 1, 546-572.
- Tourvieille, J.-N.; Larachi, F.; Duchesne, C.; Chen, J. (2017). NIR Hyperspectral Investigation of Extraction Kinetics of Ionic-Liquid Assisted Bitumen Extraction. Chem. Eng. J., 308, 1185−1199.
- Khalid F. C. (2011). Extraction of Tetrahydrofurfuryl Alcohol from Aqueous Solution Using Cyclic Solvent (C6 Ring- Containing Organic Solvent) As Industrial Solvent International Conference on Iraqi Oil Studies, 1, 81−88.
- Khalid F. C. (2008). Effect of polar component (1-propanol) on the relative volatility of the binary system n- Hexane – Benzene. Al-Khwarizmi Engineering Journal, , 4, 8-16.
- ASTM D2974-00 Method A. Standard Test Methods for Moisture, Ash, and Organic Matter of Peat and Other Organic Soils, 2000.
- Khalid F. C. (2005). New modified mixing rules for isobaric Vapor – Liquid Equilibrium (VLE) of binary and Ternary System containing 1-propanol – Hexane – Benzene using Cubic Equation of State. Jordan International Chemical Engineering Conference V (JICE05), 1, 22−29.
Citation: Khalid Farhod Chasib and Basma Mohammed Kadhim. (2022). Experimental Investigation and Feasibility Study of using Ionic Liquid for Solvent Recovery Enhancement for Extraction of Oil from the Sludge of Bottom Tank of Crude Oil. Archives of Chemistry and Chemical Engineering 4(1).
Copyright: © 2022 Khalid Farhod Chasib. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.